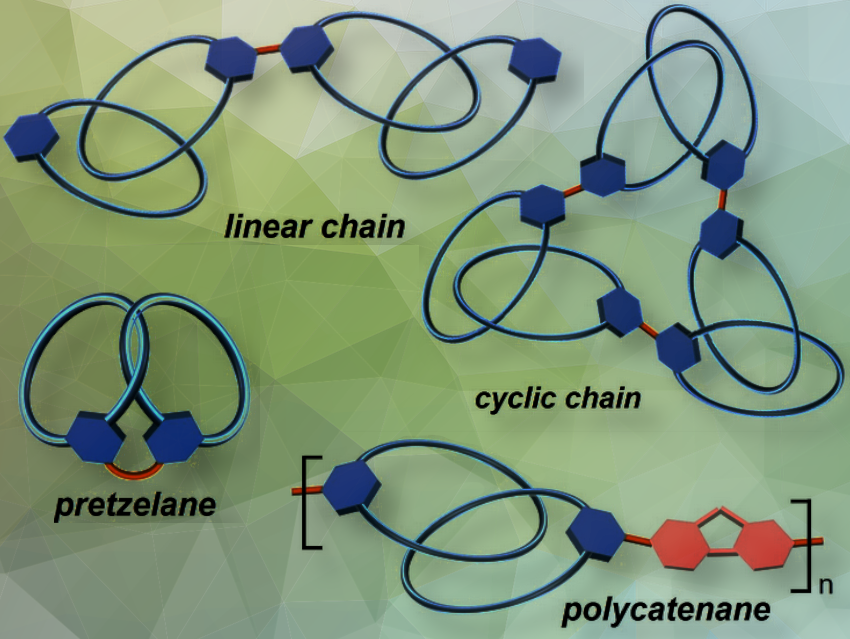
Zhi Chen, Shenzhen University, Guangdong, China, and Huan Cong, Chinese Academy of Sciences, Beijing, China, and colleagues have developed a post-functionalization strategy using iridium-catalyzed C−H borylation to modify highly strained meta-cycloparaphenylenes (mCPPs) and the mCPP-derived catenane m[9]CPP-catenane. Their approach transforms mCPP-derived macrocycles into versatile building blocks. These can be used to create new all-benzene topological structures, including linear and cyclic chains, polycatenane, and pretzelane (some pictured above).
For the prearation of the macroyclic precursers, the team mixed the mCPPs or m[9]CPP with [Ir(OMe)cod]2, 4,4′-bis(tert-butyl)-2,2′-bipyridine (dtbpy), bis(pinacolato)diboron (B2pin2), and anhydrous 1,4-dioxane, then stirred the mixtures at 80 °C for 36 h. The aryl boronic ester group in the resulting compounds was easily transformed into deuterium, vinyl, carbamate, hydroxyl, and iodide groups, providing methods to make functionalized mCPP derivatives via inter- and intramolecular C−C couplings. Examples are shown below.
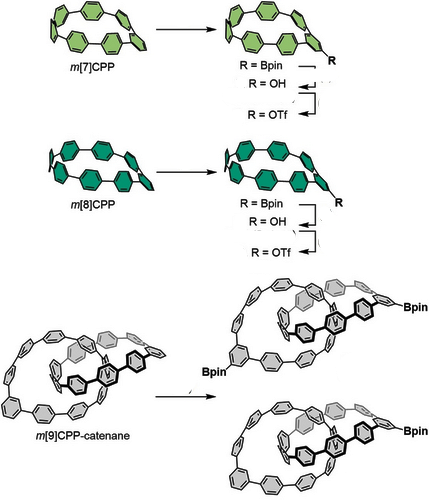
Previous work has relied on pre-functionalized building blocks to assemble all-benzene scaffolds. However, according to the researchers, their post-functionalization of strained all-benzene macrocycles streamlines synthetic routes and provides a straightforward approach to connect macrocycles in a divergent and modular fashion, opening up new possibilities for topological all-benzene structures.
- Synthesis of All-Benzene Multi-Macrocyclic Nanocarbons by Post-Functionalization of meta-Cycloparaphenylenes,
Jia-Nan Gao, An Bu, Yiming Chen, Mianling Huang, Zhi Chen, Xiaopeng Li, Chen-Ho Tung, Li-Zhu Wu, Huan Cong
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202408016