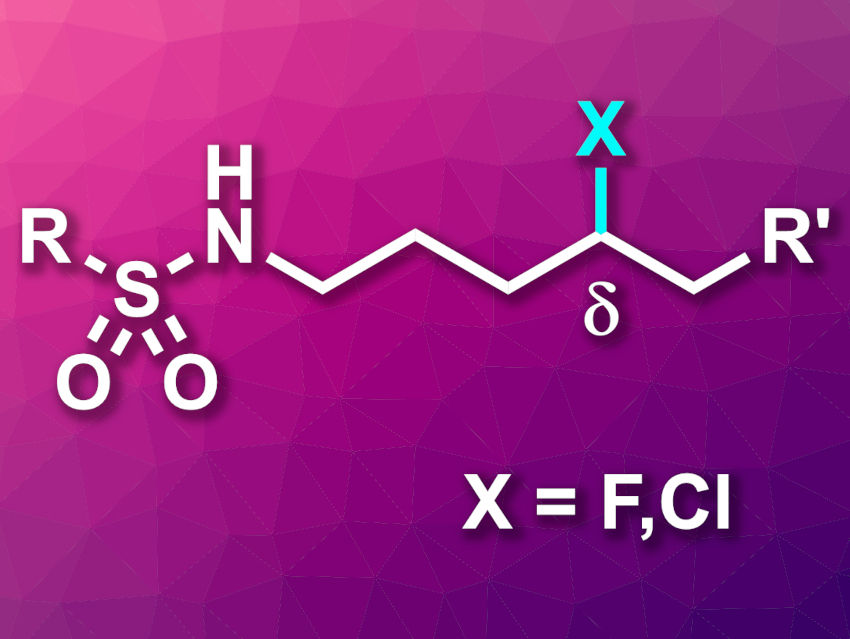
Fluorine-containing compounds play important roles, e.g., in medicinal, materials, and agrochemistry. The functionalization of C–H bonds to give C–F bonds is, thus, an interesting research target.
C(sp3)−H functionalizations via cyclometalated intermediates at the δ position (or even more remote positions) are rare. This is due to the entropically disfavored formation of larger metallacycles. Heteroatom-centered radicals could be an interesting alternative to achieve remote C(sp3)−H functionalization via 1,5-hydrogen atom transfer (1,5-HAT).
Jin-Quan Yu, Department of Chemistry, The Scripps Research Institute, La Jolla, California, USA, and colleagues have developed remote C−H fluorination and chlorination reactions of sulfonyl-protected alkyl amines via a nitrogen-centered radical precursor (general product structure pictured). The team started by synthesizing a hydrazonyl carboxylic acid radical precursor from sulfonamides in three steps. Then, they used Selectfluor as a fluorine source and observed fluorination in δ position to the sulfonamides. When they added CsF as a base and an iridium photocatalyst, the yields of the reaction were improved.
The approach can also be used to chlorinate substrates in the δ position when using ethyl trichloroacetate (ETCA) as a chlorinating reagent. Several functional groups were tolerated in the fluorination and chlorination reactions. In addition, the fluorination protocol was used on a gram scale.
- δ-C–H Halogenation Reactions Enabled by a Nitrogen-Centered Radical Precursor,
Alastair N. Herron, Ching-Pei Hsu, Jin-Quan Yu,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c01261