Metal phosphides using abundant metals such as nickel could be useful replacements for rare and expensive noble-metal catalysts in reactions that are important for a hydrogen economy, such as electrochemical hydrogen evolution or selective hydrogenations. However, the experimentally observed catalytic performance of metal phosphides has lagged behind noble metal-based catalysts so far, despite the fact that the theoretically predicted performance can be similar.
In addition, the synthesis of metal phosphide nanoparticles for catalysis can require highly reactive and potentially dangerous phosphorus sources, and methods using non-toxic precursors can need specialized support materials to achieve high dispersion of the nanoparticles. The lower stability of the metal phosphides toward oxidation compared with noble-metal nanoparticles can also be an issue.
Constanze N. Neumann, Max Planck Institute for Coal Research, Mülheim an der Ruhr, Germany, and colleagues have developed a one-step method for the preparation of small, air-stable, supported metal phosphide nanoparticles with high dispersion. The team chose triphenylphosphite (P(OPh)3) as a phosphorus precursor due to its low toxicity, air stability, and low cost, together with cheap nickel chloride hydrate as the nickel source. A reaction of these two compounds in the presence of excess hexadecylamine in 1-octadecene gave Ni2P nanoparticles with average diameters of ca. 3.7 nm. Different support materials could be added during the synthesis.
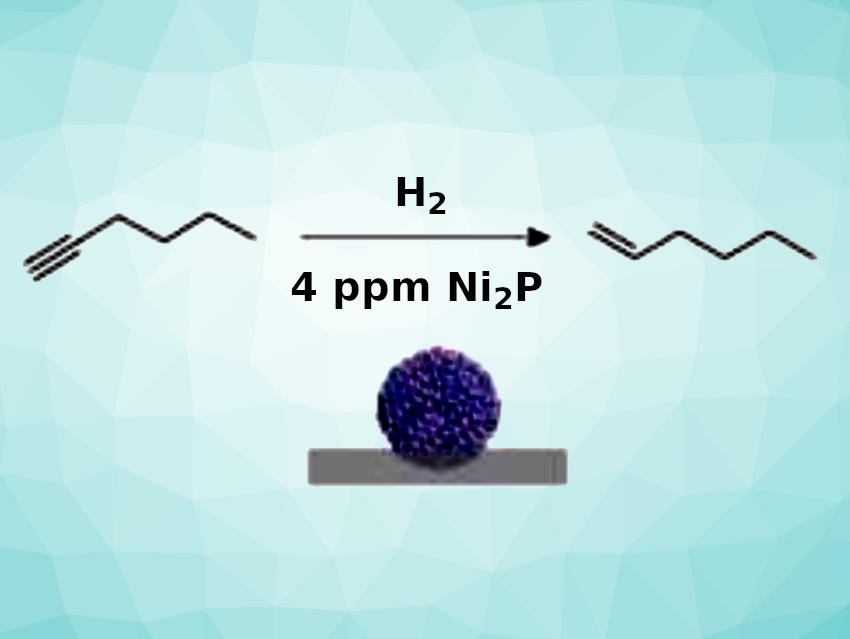
Using this approach, the team obtained a promising alumina-supported nickel phosphide catalyst (Ni2P/Al2O3 1-step). The catalyst was used for the semi-hydrogenation of 1-hexyne (pictured), and the researchers found that the performance was on par with that of the palladium-based Lindlar catalyst. A catalyst loading corresponding to 4 ppm Ni was sufficient to convert 0.1 mol of the alkyne. The stability of the catalyst was demonstrated by the fact that 85 % of the initial catalytic activity was retained after the catalyst was stored under air for 1.5 years.
- Expedited Synthesis of Metal Phosphides Maximizes Dispersion, Air Stability, and Catalytic Performance in Selective Hydrogenation,
Leila Karam, Christophe Farès, Claudia Weidenthaler, Constanze Nicole Neumann,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202404292