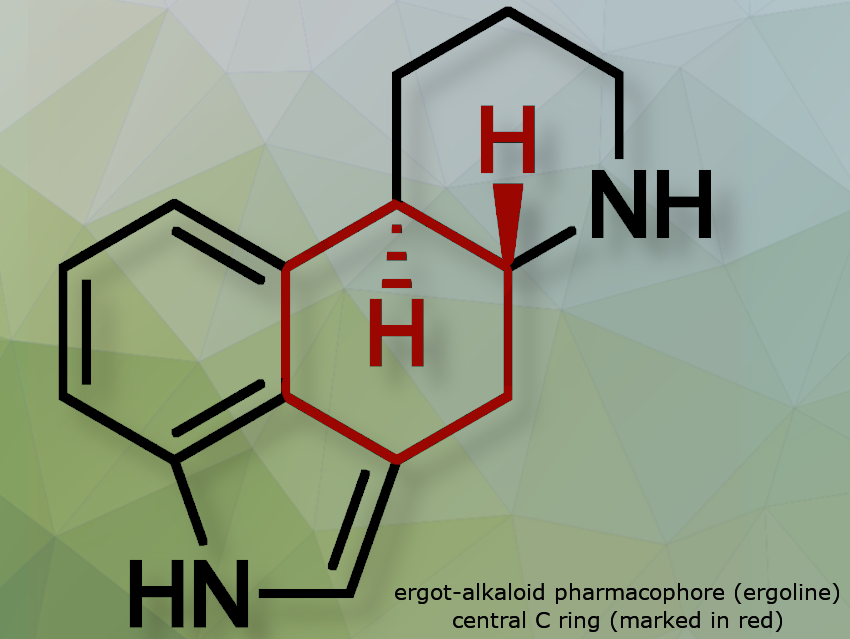
Ergot alkaloids are a class of bioactive compounds originally derived from the ergot fungus (Claviceps species), with natural and semi-synthetic variants used to treat conditions such as migraines, Parkinson’s disease, and postpartum bleeding. More than ten ergot alkaloids are clinically used, all sharing a central C ring that forms the core pharmacophore, giving them structural similarity to neurotransmitters, and enabling interaction with neurotransmitter receptors.
The haem-containing catalase chanoclavine synthase (EasC) is involved in the biosynthesis of ergot alkaloids as it catalyzes the formation of this C ring via a complex radical oxidative cyclization. Unlike typical catalases, which catalyze H₂O₂ disproportionation, EasC and its homologs belong to a broader subclass of catalases that catalyze O₂-dependent radical reactions.
Rey-Ting Guo, Hangzhou Normal University, and Hubei University, Wuhan, China, Shu-Shan Gao, Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, and National Center of Technology Innovation for Synthetic Biology, Tianjin, China, and colleagues have determined the structure and catalytic mechanism of EasC using cryo-electron microscopy. The team found a nicotinamide adenine dinucleotide phosphate (reduced) (NADPH)-binding pocket and a haem pocket common to all haem catalases, with a unique homodimeric architecture that, to their knowledge, was previously unobserved.
Unlike previous assumptions, the substrate prechanoclavine binds in the NADPH-binding pocket rather than the haem-binding pocket. The researchers also identified a slender 11.6-Å tunnel linking the haem and NADPH-binding pockets, composed mainly of hydrophobic amino acids.
In contrast to established mechanisms, EasC uses superoxide, rather than transient haem iron–oxygen complexes (such as compounds I, II, and III), to drive substrate transformation through a unique cooperative catalysis between the two pockets. The proposed mechanism for EasC involves electron transfer from prechanoclavine (PCC; intermediate) in the NADPH-binding pocket to the haem iron, reducing O₂ to Cpd III (intermediate), which decomposes to superoxide. The superoxide travels through the tunnel to the NADPH site, initiating radical oxidative cyclization of PCC, leading to decarboxylation and ring closure. The final product forms after H₂O₂ elimination.
The researchers propose that this previously unobserved enzymatic strategy may be common in metalloenzyme-catalyzed reactions and could inform the design of novel biocatalysts for pharmaceutical synthesis.
- Chanoclavine synthase operates by an NADPH-independent superoxide mechanism,
Chun-Chi Chen, Zhi-Pu Yu, Ziwei Liu, Yongpeng Yao, Peter-Leon Hagedoorn, Rob Alexander Schmitz, Lujia Yang, Lu Yu, Aokun Liu, Xiang Sheng, Hao Su, Yaqing Ma, Te Wang, Jian-Wen Huang, Lilan Zhang, Juzhang Yan, Jinping Bao, Chengsen Cui, Xian Li, Panpan Shen, Wuyuan Zhang, Jian Min, Chang-Yun Wang, Rey-Ting Guo, Shu-Shan Gao,
Nature 2025.
https://doi.org/10.1038/s41586-025-08670-3
Also of Interest

Focus: A Chemical Examination of the Isenheim Altar: Role Played in History by Horned Rye
Looking at how the Isenheim Altarpiece depicts the effects of ergot alkaloid poisoning; explaining ergot infection, the fungus’s life cycle, its potential link to the Salem witch trials, and how modern chemical knowledge ensures safe consumption of grain