The synthesis of zirconium metal-organic frameworks (MOFs) with a zeolite network is challenging because zirconium clusters typically form highly connected networks that do not align with the tetrahedral connectivity required for zeolite structures. This discrepancy makes it difficult to achieve the precise, ordered frameworks found in natural zeolites. However, overcoming these challenges is important because zeolite-like zirconium MOFs (Zr ZMOFs) can offer exceptional stability and tunable porous structures, which are valuable for applications such as gas storage, separation, and catalysis.
Hongliang Huang, Tiangong University, China, Huajun Yang, Nanjing Normal University, China, and colleagues have developed a method to synthesize Zr ZMOFs by using both ditopic and tritopic carboxylate linkers. The ditopic linker cross-link Zr6 clusters to form 4-connected zeolite-like nets. The tritopic linker creates six-membered rings (6MRs). The ring size refers to the number of T nodes in a ring.
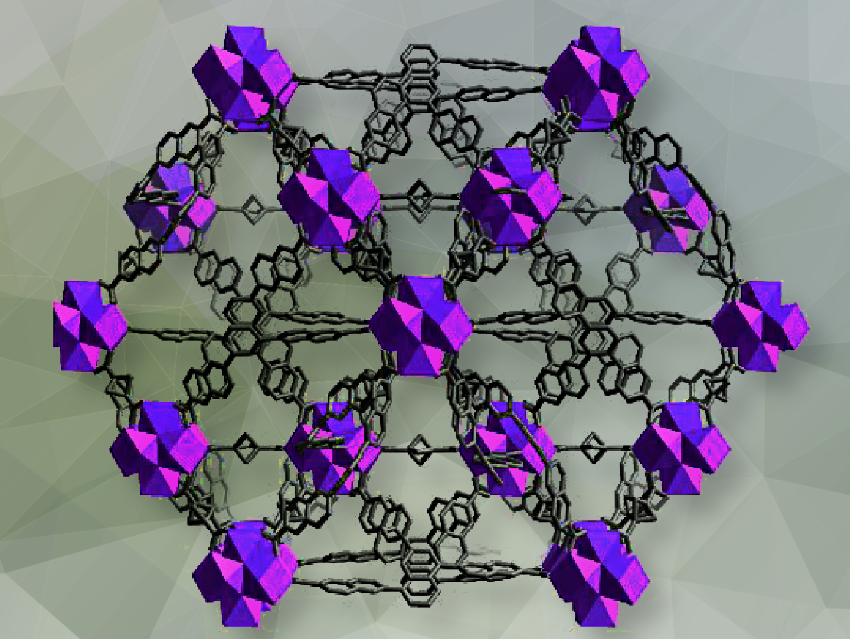
The team illustrates the feasibility of this strategy with two linker combinations to construct a Zr ZMOF, namely NNM-5. NNM stands for Nanjing Normal University Materials. NNM-5 is the first example that a zeolite sodalite (SOD; Na6(AlSiO4)6) topology has been synthesized using mixed carboxylate linkers. This means that the MOF has a similar structural arrangement to natural sodalite, which includes small and large pores formed by interconnected 6MRs.
NNM-5 ([Zr6O4(OH)4]3(NTB)2(formate)24(H2O)12·6Cl·(solv)x) was synthesized with the solvothermal reaction of ZrCl4 and H3NTB (4,4′,4”-nitrilotribenzoic acid) in the presence of formic and hydrochloric acids in DMF at 120 ºC for 3 days. The team has extended the 6MR strategy to synthesize another non-zeolitic MOF: NNM-6. NNM-6 (pictured above; Zr6O4(OH)4(BTN)2(BCP)3·(solv)x) was synthesized by heating H3BTN (6,6′,6”-(1,3,5-Benzenetriyl)tris[2-naphthalenecarboxylic acid]) and H2BCP (bicyclo[1.1.1]pentane-1,3-dicarboxylic acid) with ZrCl4 in the presence of formic acid in DMF at 120 ºC for 4 days.
NNM-5 effectively separates C6 isomers by adsorbing linear n-hexane (nC6) with high capacity while excluding branched isomers like 3-methylpentane (3MP) and 2,2-dimethylbutane (22DMB), thanks to its unique pore structure that creates a significant energy barrier for larger, branched molecules. The material demonstrated high selectivity in separating nC6 from its branched counterparts in both single-component and multi-component tests, maintaining excellent performance and structural stability over multiple cycles.
Considering that 6MR is a prominent structural building block in many zeolites, the researchers think that it is possible to extend this strategy to other zeolite structures including zeolite A and Y by carefully selecting the carboxylate ligands. According to the researchers this highlights the effectiveness of their approach for advanced material synthesis and separation applications.
- Six-Membered Ring Directed Assembly of a Zirconium Zeolitic Metal-Organic Framework for Efficient Separation of Hexane Isomers,
Lei Gan, Beibei Sun, Chaozhuang Xue, Zhi Fang, Zongjing Xiao, Suyun Deng, Pengfei Li, Hongliang Huang, Huajun Yang,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202412494