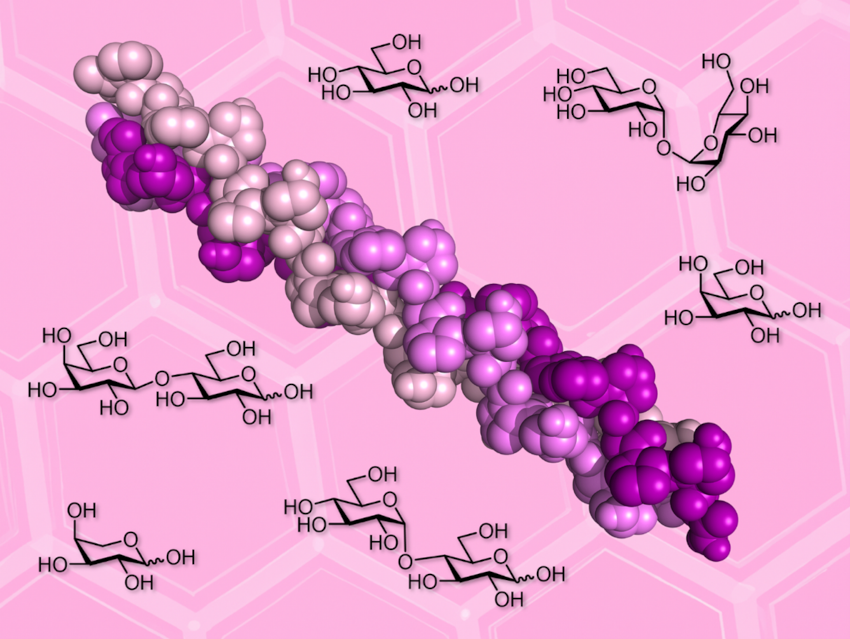
At high concentrations, sugars can stabilize globular proteins against heat, freezing, and desiccation. Many extremophiles (organisms that live in extreme environments) produce large amounts of sugars to survive harsh conditions. Sugar additives can also be useful for stabilizing biomolecules during storage. The effects of sugar co-solutes on fibrillar proteins such as collagen are, however, underexplored.
Tomas Fiala, Helma Wennemers, Swiss Federal Institute of Technology (ETH) Zurich, Switzerland and colleagues have studied the stability of collagen triple helices derived from a collagen model peptide (pictured) in solutions of six common sugars. The team chose arabinose (Ara), galactose (Gal), glucose (Glc), lactose (Lac), maltose (Mal), and trehalose (Tre). They studied the thermal stability of the triple helix in different sugar solutions using circular dichroism (CD) spectroscopy as a monitoring tool.
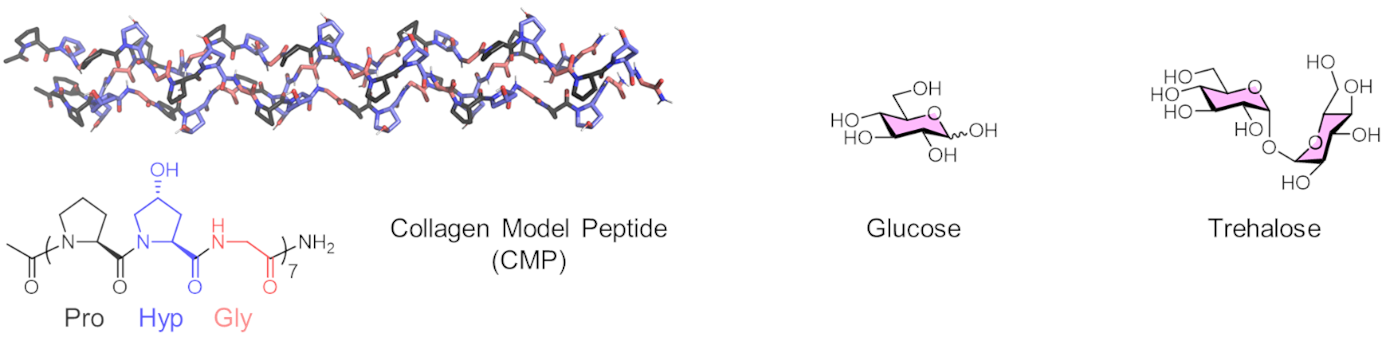
The researchers found that sugars stabilize the collagen triple helix in a concentration-dependent manner, increasing its melting temperature by as much as 17 °C. The team observed a similar stabilizing effect for all examined sugars. For instance, a 1 M solution of the disaccharide trehalose had roughly the same effect as a 2 M solution of the monosaccharide glucose. These findings are surprising as trehalose is known for its superior bioprotection of globular proteins. The work calls for further studies of the effects of sugar co-solutes on fibrillar proteins and could be useful for the choice of storage conditions for collagen preparations.
- Carbohydrate Co‐Solutes Stabilize Collagen Triple Helices,
Kota Nomura, Tomas Fiala, Helma Wennemers,
ChemBioChem 2024.
https://doi.org/10.1002/cbic.202300860