Cane sugar was the first organic natural product that was isolated in pure crystalline form. Around 600 AD, it was first isolated by the Persians by evaporating aqueous extracts of East Asian sugar cane (Saccharum officinarum). Arab merchants brought this exotic specialty to Europe in the early Middle Ages.
Andreas Sigismund Marggraf, a member of the Royal Academy of Berlin, Germany, recognized that sugar could also be isolated from the sugar beet plant Beta vulgaris, which grows in Europe. The cane sugar monopoly was, thus, broken when the world’s first beet sugar factory was built in 1801 [1].
Today, in addition to cane and other sugars, there are plenty of artificial sweeteners. In the quiz, we take a closer look at the chemistry of the sugary world.

Which molecule is referred to in everyday speech as “sugar”?
- D-Glucose
- Sucrose or O-α-D-glucopyranosyl-(1→2)-β-D-fructofuranoside
- Saccharin
- Sucralose
See answer
2. Sucrose or O-α-D-glucopyranosyl-(1→2)-β-D-fructofuranoside is correct. It is a sugar composed of glucose and fructose subunits and is found in many plants, including sugar cane and sugar beets.
1. D-Glucose is the most abundant monosaccharide. It is created during photosynthesis.
3. Saccharin is an artificial sweetener with a chemical composition unrelated to sugars. It is derived from the aromatic hydrocarbon benzoic sulfimide.
4. Sucralose is also an artificial sweetener, but it is a chlorinated derivative of sucrose. Three chlorine atoms replace three of the hydroxy groups—in the C1 and C6 positions of fructose and the C4 position of glucose—to give a 1,6-dichloro-1,6-dideoxyfructose–4-chloro-4-deoxygalactose disaccharide, making it much sweeter than sucrose and non-caloric.

What is the chemical name of the artificial sweetener commonly known as “aspartame”?
- Thaumatin
- L-α-Aspartyl-L-phenylalanine methyl ester
- Aspartic acid
- Acesulfame potassium
See answer
2. Aspartame is L-α-Aspartyl-L-phenylalanine methyl ester.
1. Thaumatin is a low-calorie sweetener.
3. Aspartic acid is also called 2-aminobutanedioic acid and is an α-amino acid that is used in the biosynthesis of proteins.
4. Acesulfame potassium is also known as acesulfame K or ace K, and is an artificial sweetener [1].
Reference
[1] Klaus Roth, Erick Lück, The Saccharin Saga – Part 7, ChemistryViews 2016. https://doi.org/10.1002/chemv.201600034

What is not a sweetener?
- Saccharin
- Aspartame
- Acesulfame-K
- Butylated Hydroxyanisole (BHA)
- Sucrose
- Splenda
See answer
4. Butylated Hydroxyanisole (BHA) is an antioxidant.
1. Saccharin is an artificial sweetener with a chemical composition unrelated to sugars. It is derived from the aromatic hydrocarbon benzoic sulfimide.
2. Aspartame is L-α-Aspartyl-L-phenylalanine methyl ester and an artificial sweetener.
3. Acesulfame-K or acesulfame potassium or ace K is an artificial sweetener.
5. Sucrose or O-α-D-glucopyranosyl-(1→2)-β-D-fructofuranoside is a sugar composed of glucose and fructose subunits and is found in many plants, including sugar cane and sugar beets.
6. Splenda is also called sucralose and is also an artificial sweetener, it is a chlorinated derivative of sucrose.

What is better not to use to sweeten your coffee?
- Potassium 6-methyl-2,2-dioxo-2H-1,2λ6,3-oxathiazin-4-olate
- Lead(II) acetate
- Lysergic acid diethylamide
- Acesulfame potassium
See answer
2. and 3. are not recommended:
2. Lead(II) acetate was used as a sweetener by the ancient Romans but causes lead poisoning [1], and
3. lysergic acid diethylamide, better known as LSD, is a powerful hallucinogenic drug [2].
The other two options, 1. and 4., are both acesulfame-K, also known as acesulfame potassium or potassium 6-methyl-2,2-dioxo-2H-1,2λ6,3-oxathiazin-4-olate. It is a highly successful product, primarily due to its high stability and, most importantly, its synergistic effect when combined with other sweeteners, especially aspartame. This calorie-free sugar substitute is marketed under the trade names Sunett and Sweet One. In the European Union, it is recognized by the E-number E950 [2].
Reference
[1] Klaus Roth, Erick Lück, The Saccharin Saga – Part 4, ChemistryViews 2016.
[2] 75th Anniversary: Lysergic Acid Diethylamide (LSD), ChemistryViews 2013.
[3] Klaus Roth, Erick Lück, The Saccharin Saga – Part 7, ChemistryViews 2016. https://doi.org/10.1002/chemv.201600034

Sucralose (Splenda®) is a chlorinated derivative of which sugar?
- Saccharin
- Glycogen
- Ribitol ((2R,3S,4S)-pentane-1,2,3,4,5-pentol)
- Sucrose
See answer
4. Sucrose, which was once known as saccharose, is correct.
1. Saccharin is an artificial sweetener with a chemical composition unrelated to sugars. It is derived from the aromatic hydrocarbon benzoic sulfimide.
2. Glycogen is the stored form of glucose that is made up of many connected glucose molecules. In humans the majority of glycogen is stored in skeletal muscles and the liver.
3. Ribitol ((2R,3S,4S)-pentane-1,2,3,4,5-pentol) or adonitol, is a crystalline pentose alcohol (C5H12O5) formed by the reduction of ribose. Ribose is a five-carbon sugar found in RNA (ribonucleic acid).
Reference
Klaus Roth, Erick Lück, The Saccharin Saga – Part 9, ChemistryViews 2016. https://doi.org/10.1002/chemv.201600059

Which artificial sweetener was initially discovered by a chemist who noticed its sweet taste when he accidentally spilled some on his finger?
- Aspartame
- Cyclamate
- Saccharin
- Steviol glycosides
See answer
1. Aspartame is correct.
James Schlatter, a chemist with the firm G. D. Searle & Company, Skokie, IL, USA, was tasked with finding new possibilities for treating stomach ulcers. In the context of this project, in 1965, he decided to prepare the tetrapeptide H-Trp-Met-Asp-Phe-OH. This particular amino acid sequence corresponds to the C-terminus of the enzyme gastrin, which, among other things, intensely stimulates the production of stomach acid. During the course of recrystallizing the compound, he spilled something on his finger and only this led to the discovery that the substance is sweet.
Reference
Klaus Roth, Erick Lück, The Saccharin Saga – Part 6, ChemistryViews 2016. https://doi.org/10.1002/chemv.201600030

True or False: Some artificial sweeteners can have a cooling effect on the tongue when dissolved in water.
See answer
That’s correct.
Erythritol, for example, has a strong cooling effect when it dissolves in water (i.e., an endothermic process/one with a positive heat of solution), which is often compared with the cooling effect of mint flavors. The cooling effect is present only when erythritol is not already dissolved in water, a situation that might be experienced in an erythritol-sweetened frosting, chocolate bars, chewing gum, or hard candy.
A similar cooling effect is seen in xylitol. Xylitol is used as a sugar substitute in manufactured products such as drugs, dietary supplements, confections, toothpaste, and chewing gum, but is not a common household sweetener.
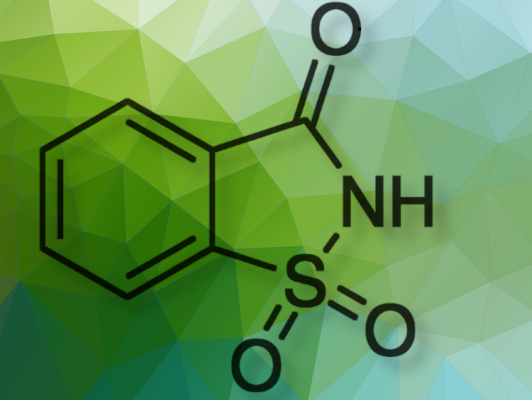
From which molecule did the first industrial saccharin synthesis begin in 1879?
- Toluene
- Sucrose
- Ethylene glycol
- Methyl anthranilate
See answer
1. Toluene is correct.
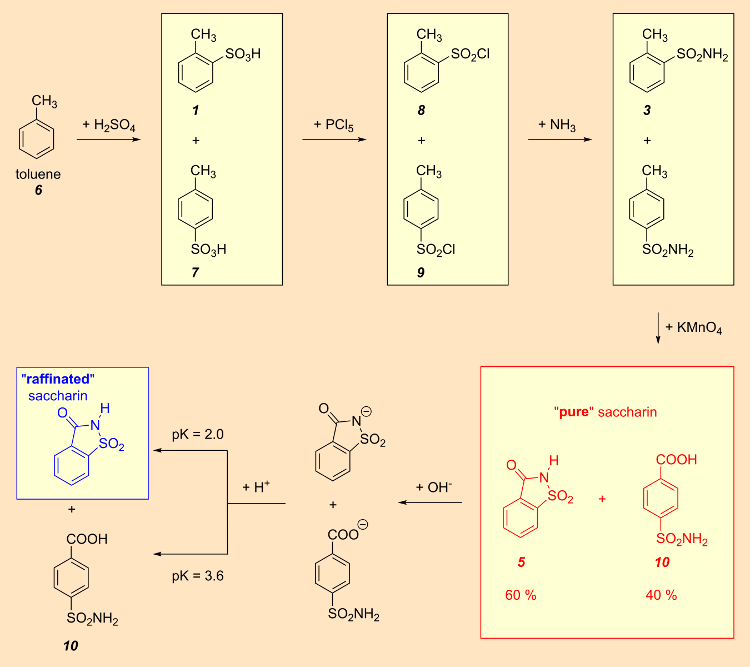
The sulfonation of toluene with sulfuric acid resulted in a mixture of ortho– and para-toluenesulfonic acids (pictures below). This mixture was then converted to the corresponding sulfonyl chlorides with phosphorus pentachloride. Separation of the two isomers seemed straightforward, since ortho-toluenesulfonyl chloride is liquid, while the corresponding para compound is solid. In the early years of production, no one realized that the resulting saccharin was actually 40 % contaminated with p-sulfamoylbenzoic acid [1].
4. In 1950, an improved synthesis was developed where methyl anthranilate successively reacts with nitrous acid (from sodium nitrite and hydrochloric acid), sulfur dioxide, chlorine, and then ammonia to yield saccharin.
2. The disaccharide sucrose is a sugar composed of glucose and fructose subunits.
3. Ethylene glycol is an organic compound with the formula (CH₂OH)₂ which is used as an antifreeze and in coolants, detergents, paints, lacquers, pharmaceuticals, adhesives, and cosmetics.
Reference
[1] Klaus Roth, Erick Lück, The Saccharin Saga – Part 2, ChemistryViews 2016. https://doi.org/10.1002/chemv.201500087

What is healthier?
- A beverage sweetened with aspartame
- A beverage sweetened with sugar
See answer
The answer is not straightforward. Water would be healthier in any case.
Switching to foods and beverages with less sugar but still retaining sweetness is a practical way to reduce sugar and calorie intake.
The International Agency for Research on Cancer (IARC) of the World Health Organization (WHO) has classified the sweetener aspartame as “possibly carcinogenic” for humans. The WHO Expert Committee on Food Additives (JECFA) reiterated the previously permitted daily intake of 40 mg of aspartame per kilogram of body weight [1,2].
“Since aspartame is broken down in the intestine into harmless or quantitatively harmless cleavage products and does not pass intact into the body, there is not much likelihood of a causal relationship between aspartame intake and cancer risk, even from a purely mechanistic point of view,” said Dr. Stefan Kabisch, study physician in the Department of Endocrinology and Metabolic Medicine (German Center for Diabetes Research / DZD), Campus Benjamin Franklin (CBF), Charité – Universitätsmedizin Berlin, Germany.
In contrast, there is substantial evidence suggesting that sugar consumption contributes to obesity, type 2 diabetes, tooth decay, and consequently, an increased risk of cancer. Switching from sweeteners to sugar would likely increase the risk of developing these diseases.
To advance our understanding, further research with robust methodology, particularly large-scale human clinical intervention studies on various sweeteners, is needed. The International Agency for Research on Cancer (IARC) and the Joint FAO/WHO Expert Committee on Food Additives (JECFA) support further research to provide more precise recommendations in the future.
References
[1] World Health Organization, Summary of findings of the evaluation of aspartame at the International Agency for Research on Cancer (IARC) Monographs Programme’s 134th Meeting, 6–13 June 2023 and The Joint FAO/WHO Expert Committee On Food Additives (JECFA) 96th meeting, 27 June–6 July 2023.
[2] Elio Riboli, Frederick A. Beland, Dirk W. Lachenmeier, M. Matilde Marques, David H. Phillips, Eva Schernhammer, Abdul Afghan, Ricardo Assunção, Giovanna Caderni, J. Christopher Corton, Gisela de Aragão Umbuzeiro, Daphne de Jong, Melanie Deschasaux-Tanguy, Allison Hodge, Junko Ishihara, Dan D. Levy, Daniele Mandrioli, Marjorie L McCullough, Sarah A. McNaughton, Takeshi Morita, Anne P Nugent, Kumiko Ogawa, Arun R. Pandiri, Consolato M. Sergi, Mathilde Touvier, Luoping Zhang, Lamia Benbrahim-Tallaa, Shirisha Chittiboyina, Danila Cuomo, Nathan L. DeBono, Charlotte Debras, Aline de Conti, Fatiha El Ghissassi, Emma Fontvieille, Rhea Harewood, John Kaldor, Heidi Mattock, Elisa Pasqual, Gabrielle Rigutto, Hannah Simba, Eero Suonio, Susana Viegas, Roland Wedekind, Mary K. Schubauer-Berigan, Federica Madia, Carcinogenicity of aspartame, methyleugenol, and isoeugenol, The Lancet Oncology 2023, 24(8), 848-850. https://doi.org/10.1016/S1470-2045(23)00341-8
Reference
[1] Klaus Roth, Erich Lück, The Saccharin Saga – Part 1, ChemistryViews 2015. https://doi.org/10.1002/chemv.201500076
Also of Interest
Sugar Chemistry
We all love sugar, but what is it chemically and since when do we know sugar?

The Saccharin Saga
The invention of the first artificial sweetener and a lifetime battle for credit




It is highly informative and explained nicely
Thanks