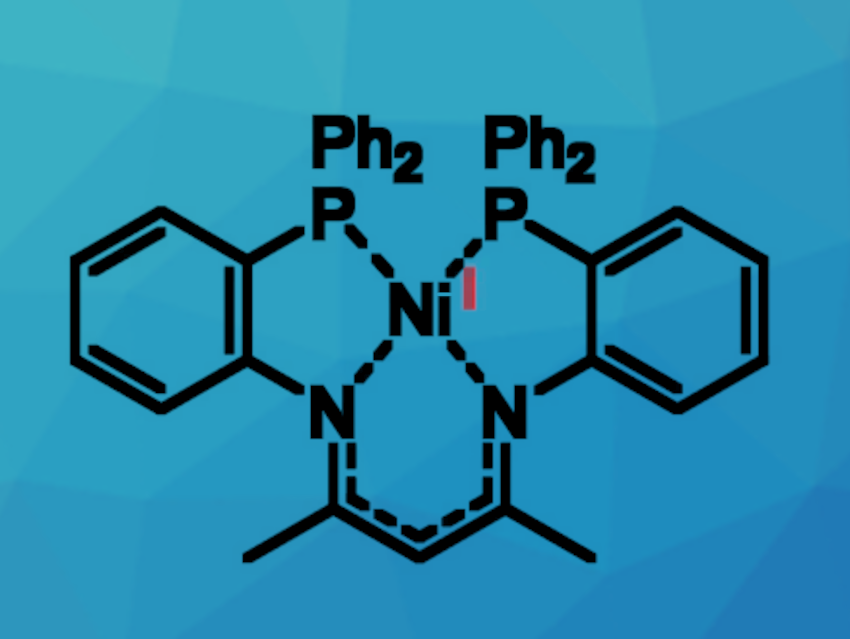
Ni(I) compounds are interesting research targets, mainly due to their unique reactivity. They could, for example, have potential uses in catalysis and small-molecule activation—with the possibility of replacing expensive palladium or platinum. Peter W. Roesky, Karlsruhe Institute of Technology (KIT), Germany, and colleagues have found that a bis(diphenyl)-phosphine-functionalized ß-diketimine ligand (PNac-H) can be used for the synthesis of a subvalent Ni(I) complex [PNac-Ni] (pictured above).
The ligand was obtained via a two-step synthesis route. First, a fluorine-functionalized precursor (FNac-H) was formed by a condensation reaction between acetylacetone and two equivalents of 2-fluoroaniline. FNac-H was then reacted with KPPh2 to obtain the desired bis-phosphine β-diketimine ligand PNac-H. Treatment of this ligand with [Ni(cod)2] (cod = cyclooctadiene) resulted in the formation of the nickel(I) complex [PNac-Ni].
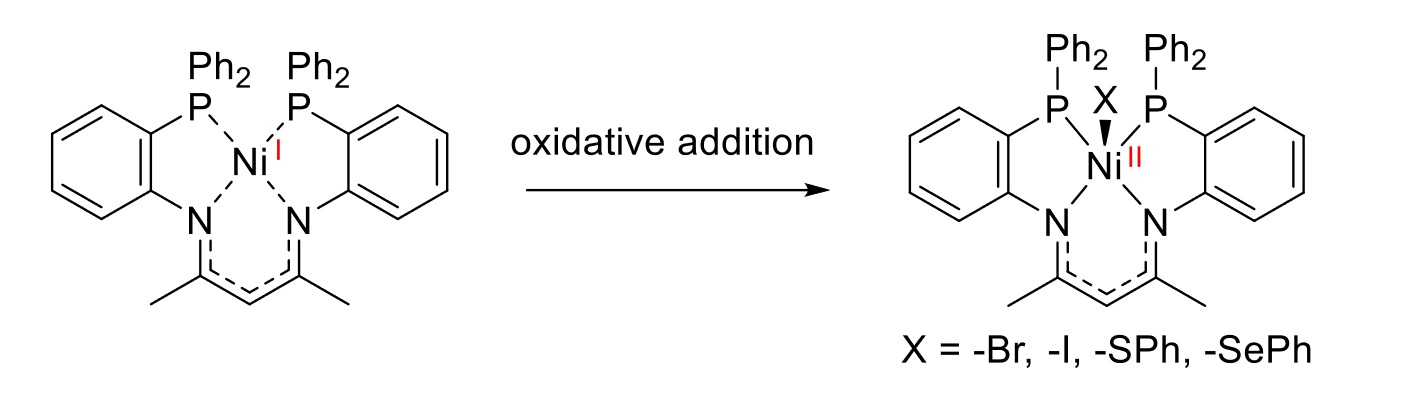
The team then investigated the Ni(I) complex with regard to its reactivity in the activation of small molecules (pictured below). The complex allowed the activation of different substrate classes, such as CH2X2 (X = Br, I), I2, or Ph2E2 (E = S, Se). According to the researchers, the ligand stabilizes the reactive Ni(I) species while, at the same time, enabling activation processes due to a hemilabile coordination behavior and an accessible axial coordination site.
- A Phosphine‐ß‐diketiminate Nickel(I)‐Complex for Small Molecule Activation,
Christina Zovko, Frederic Krätschmer, Sarah Schmidt, Tim P. Seifert, Michael T. Gamer, Peter Werner Roesky,
ChemPlusChem 2022.
https://doi.org/10.1002/cplu.202200288