Recently, there has been a small renaissance in the study of beryllium chemistry. However, our understanding of how the size and electronic properties of ligands affect the structure and reactivity of beryllium complexes remains limited.
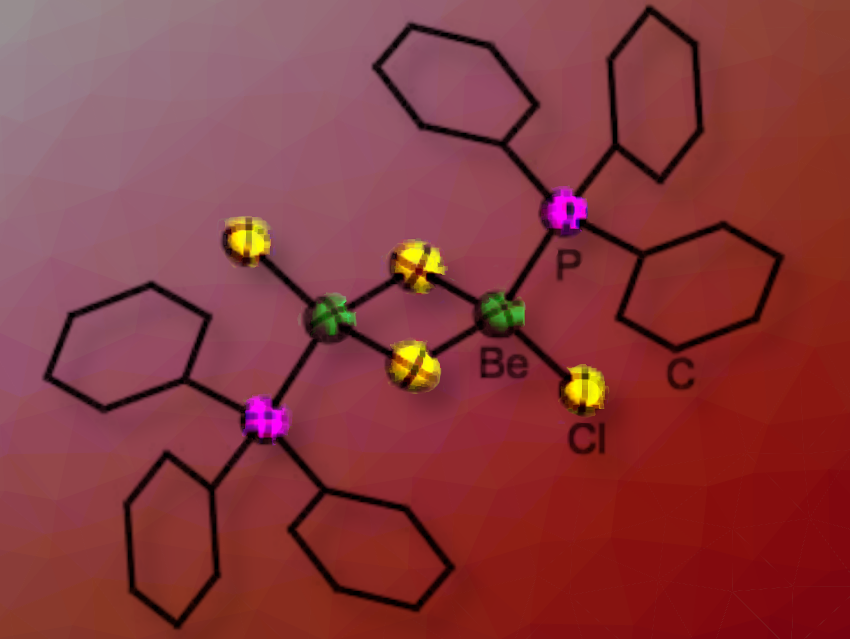
Magnus R. Buchner and Sergei I. Ivlev, University of Marburg, Germany, have prepared a series of phosphine complexes of beryllium chloride, bromide, and iodide, [(PMe2Ph)2BeX2], [(PMePh2)2BeX2] and [(PPh3)BeX2]2 (X = Cl, Br, I). The advantage of phosphines is that their electronic and steric properties are very well investigated and can be described by their Tolman electronic parameters and their cone angles.
These complexes showed that if the cone angle of the phosphine is smaller than 136° to 145°, two ligands can coordinate to a beryllium dihalide unit (136° (PMePh2) and 145° (PPh3)), while only one ligand is accommodated when the cone angle is larger (PPh3)BeCl2]2. Ligand dissociation occurs in these complexes and the under-coordinated beryllium centre can activate chlorinated solvents. If the ligands are very electron donating they react with chlorinated solvents and form phosphonium salts. In contrast, less Lewis basic phosphines only act as spectator ligands and solely halide-chloride exchange occurs at the beryllium centre.
- Steric influence on the constitution of beryllium phosphine complexes,
Magnus Richard Buchner, Sergei I. Ivlev,
European Journal of Inorganic Chemistry 2023.
https://doi.org/10.1002/ejic.202300199