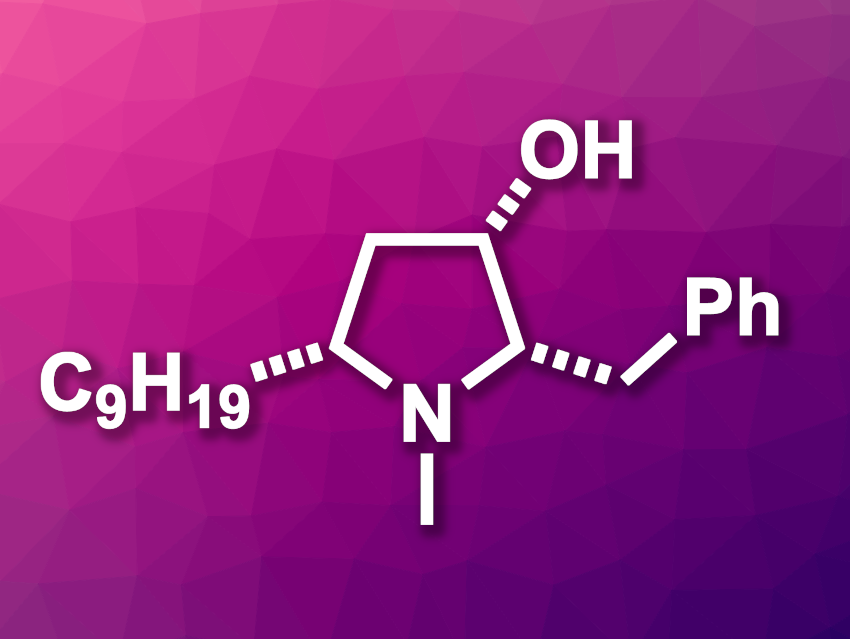
Pyrrolidines, or tetrahydropyrroles, are heterocyclic units often found, e.g., in natural products. 3-Hydroxypyrrolidines, for example, are part of several pharmaceutically active compounds. (+)-Preussin (pictured), which was isolated from a fungus, is a functionalized 3-hydroxypyrrolidine. Preussin derivatives have shown, e.g., antifungal, antibacterial, and antiviral properties. Thus, the synthesis of enantiomerically pure (+)-preussin is an interesting research target.
Jae-Sang Ryu, Ewha Womans University, Seoul, Republic of Korea, and colleagues have developed a method for the stereoselective total synthesis of (+)-preussin. The team started from commercially available ethyl-D-3-phenyl lactate, which was converted to a sulfamate ester tethered to an allylic alcohol. This intermediate underwent a gold(I)-catalyzed cyclization to obtain a six-membered cyclic sulfamidate. The team then used a cross-metathesis reaction to introduce the long alkyl substituent, followed by a hydrogenation/deprotection step.
An intramolecular Mitsunobu reaction was used to convert the cyclic sulfamidate into an azabicyclic sulfamidate (a 3-oxa-2-thia-1-azabicyclo[2.2.1]heptane derivative). This intermediate underwent a stereospecific ring-opening to obtain the desired 3-hydroxypyrrolidine scaffold. Deprotection and methylation steps then led to (+)-preussin. According to the researchers, the developed approach could be useful for the synthesis of other hydroxypyrrolidine derivatives, which could enable studies of their structure–activity relationship.
- Sulfamidate-Based Stereoselective Total Synthesis of (+)-Preussin Using Gold(I)-Catalyzed Intramolecular Dehydrative Amination: Dead End and Detour,
Yunjeong Park, Jae-Sang Ryu,
J. Org. Chem. 2023.
https://doi.org/10.1021/acs.joc.3c00670