Organic molecules with multiple readily accessible redox states are interesting research targets. However, organic radicals are generally highly reactive, and thus, they can be challenging to synthesize. Exploiting the full potential of such species requires the isolation of stable forms.
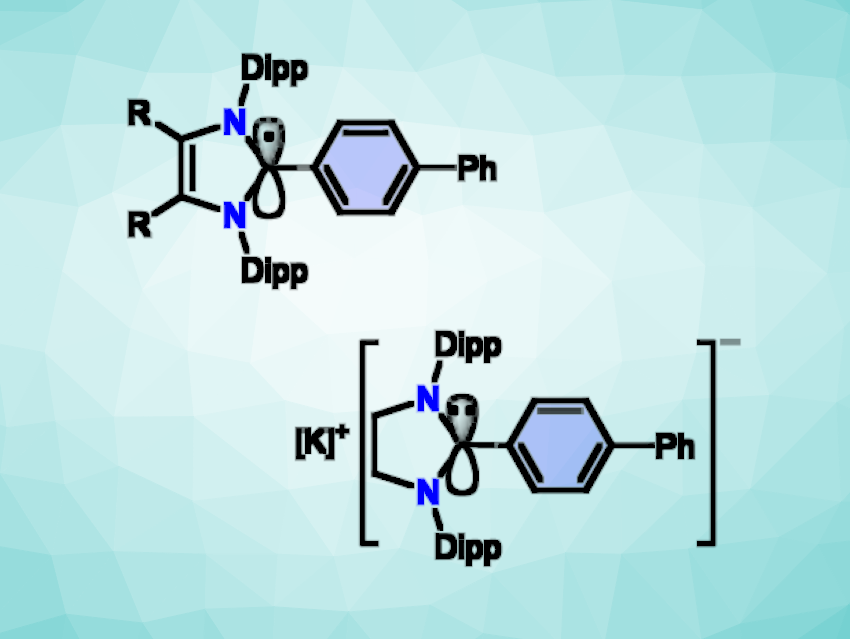
Rajendra S. Ghadwal, University of Bielefeld, Germany, Diego M. Andrada, Universität des Saarlandes, Saarbrücken, Germany, and colleagues have prepared radicals and anions based on classical N-heterocyclic carbenes (NHCs). The products (pictured above, R = H, Me) are accessible as crystalline solids.
The team first prepared C2-biphenylated 1,3-imidazoli(ni)um bromide starting materials via a direct C2-arylation of NHCs with 4-bromobiphenyl using a nickel cyclooctadiene catalyst. The researchers then found that reductions of the 1,3-imidazoli(ni)um bromides with KC8, a one-electron reducing agent, yielded radicals and anions (simplified structure pictured below). The radicals and anions are stabilized by delocalization of the unpaired or lone-pair electron, respectively, over the C2-biphenyl group.
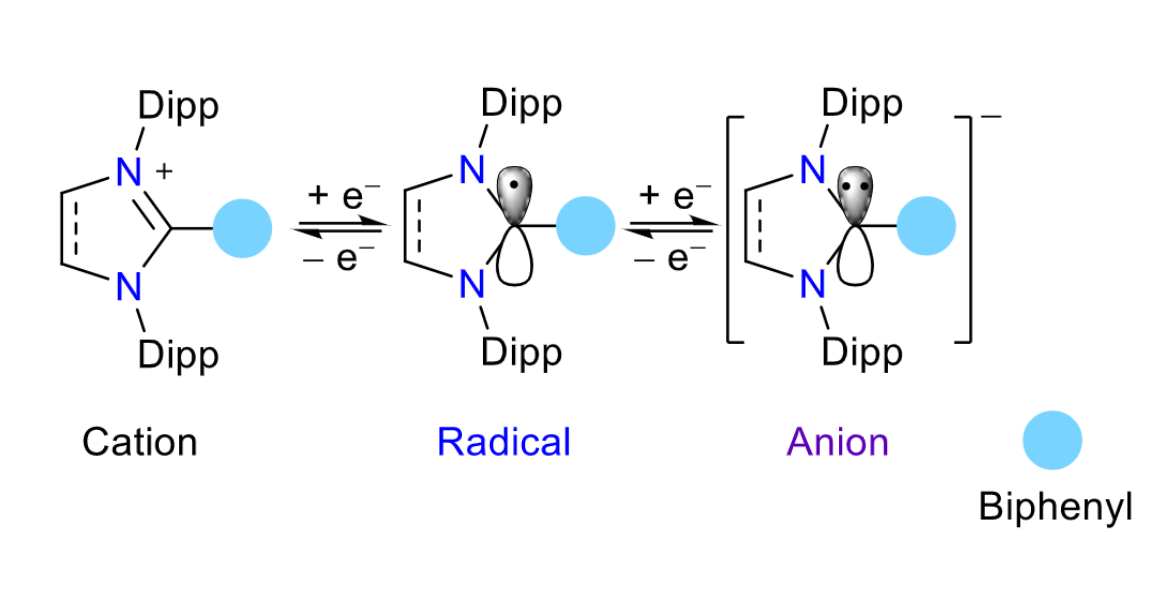
In summary, a biphenyl substituent at the C2-position of NHCs boosts the stability of the corresponding radicals and introduces an additional anionic redox state. Such compounds with stable multiple redox states are promising for the development of advanced functional materials, according to the researchers.
- Crystalline Anions Based on Classical N‐Heterocyclic Carbenes,
Arne Merschel, Dennis Rottschäfer, Beate Neumann, Hans-Georg Stammler, Mark Ringenberg, Maurice van Gastel, T. Ilgin Demirer, Diego M. Andrada, Rajendra Ghadwal,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202215244



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)