Kinetically stabilized germylenes are known to react with a chalcogen source to give the corresponding heavy ketones. Although some chalcogenation reactions have also been performed for nitrogen ligand-substituted germylenes, the resulting products have been limited to heavy urea-type compounds and those thermodynamically stabilized through coordination with a Lewis base or donor-acceptor type interaction. Hence, heavy germaneamides have not yet been isolated, so far.
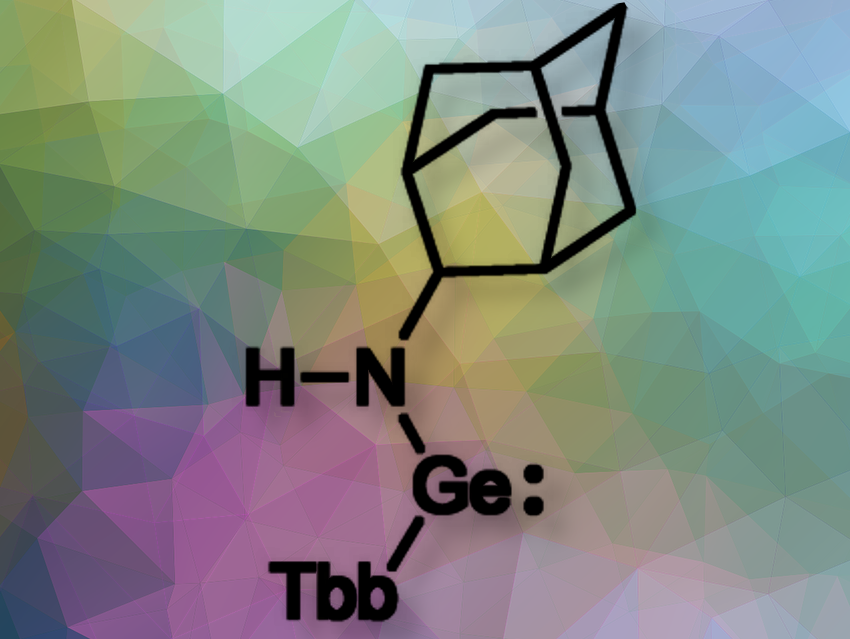
Norihiro Tokitoh, ICR, Kyoto University, Japan, and colleagues have synthesized and isolated 1-adamantylamino(aryl)germylene (pictured) bearing an extremely bulky aryl group (Tbb = 2,6-bis[bis(trimethylsilyl)methyl]-4-t-butylphenyl) on the germanium atom. The isolated 1-adamantylamino(aryl)germylene was found to be stable in toluene solution even at 110°C for three days without decomposition.
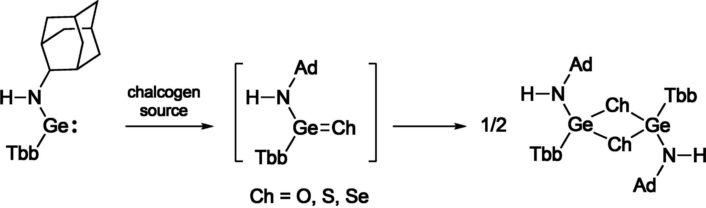
The researchers carried out chalcogenation reactions of the resulting 1-adamantylamino(aryl)germylene to synthesize the corresponding heavy amides. Instead of the expected monomeric germaneamides, the corresponding trans-1,3,2,4-dichalcogenadigermetanes were obtained, suggesting that a bulkier ligand is required to isolate the germaneamides as stablecompounds.
- Synthesis of 1‐Adamantylaminogermylene and Its Chalcogenation Reactions,
Mariko Yukimoto, Kazuaki Kanda, Takamasa Aoki, Yoshiyuki Mizuhata, Norihiro Tokitoh,
European Journal of Inorganic Chemistry 2024.
https://doi.org/10.1002/ejic.202300663