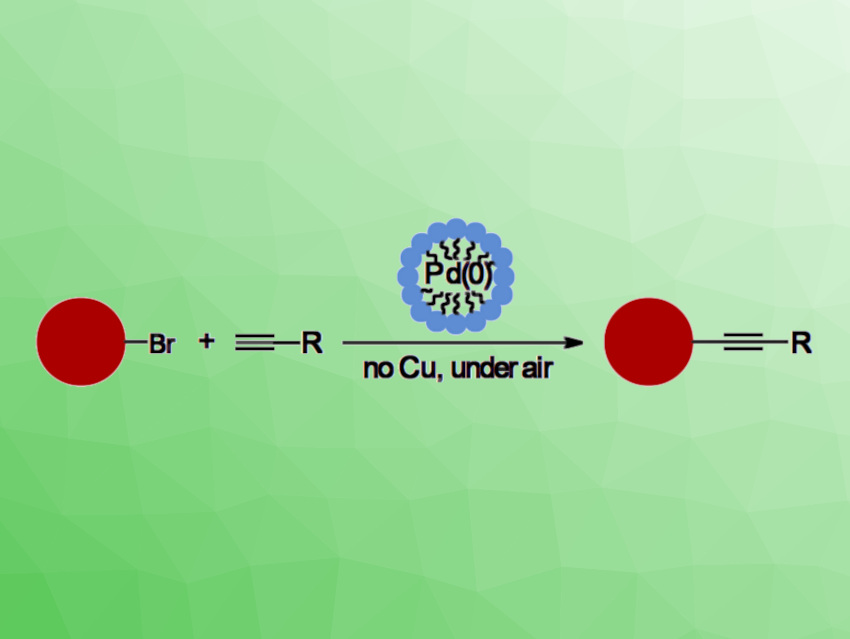
The Sonogashira coupling is a widely used carbon–carbon bond-forming reaction, providing direct, high-yield access to alkynes with good functional group tolerance. The method generally requires the use of an inert atmosphere to protect the palladium catalyst, and it needs a copper-based co-catalyst.
Sara Mattiello, University of Milano-Bicocca, Milan, Italy, and colleagues have developed a copper-free method to perform Sonogashira coupling reactions in water and under air (general reaction pictured). They took advantage of the capability of the industrial surfactant Kolliphor EL to form oxygen-free compartments in aqueous media. The reactions can run smoothly even at room temperature.
The team demonstrated the generality of the approach by preparing several (hetero)aryl alkynes in good to excellent yields, including examples of organic luminescent materials. The approach qualifies as a sustainable methodology, with improved E-factors (environmental factors, ratio of the mass of waste per mass of product) compared with standard protocols using organic solvents.
- Efficient Copper‐Free Sonogashira Coupling in Water and under Ambient Atmosphere,
Erika Ghiglietti, Elena Aurora Incarbone, Sara Mattiello, Luca Beverina,
Eur. J. Org. Chem. 2024.
https://doi.org/10.1002/ejoc.202400223