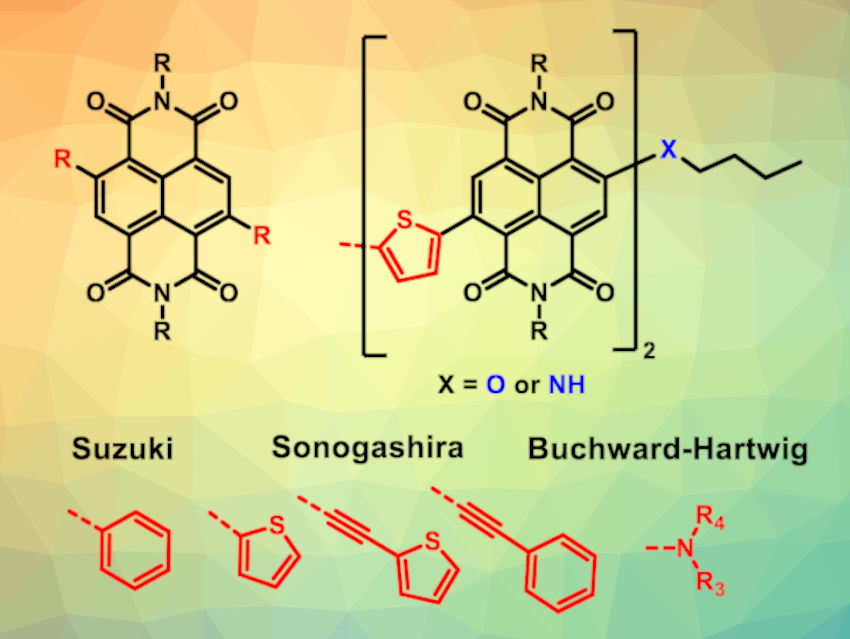
Optimizing the efficiency of organic photovoltaic cells (oPVCs) is an important research target for the improved production of renewable energy. However, the environmental impact of their synthesis has been less well studied. Core-functionalized naphthalene diimides (c-NDIs) are a significant component in oPVCs and organic electronics.
Barnaby William Greenland, University of Sussex, Brighton, UK, and colleagues have developed a mechanochemical synthesis of core-functionalized naphthalene diimides (general structure pictured above on the left) using a vibratory ball mill. The team prepared c-NDIs with a wide structural variety using ball milling in the presence of Pd catalysts to functionalize N,N‘–bis(2–ethylhexyl)–2,6–dibromo–1,4,5,8–naphthalenetetracarboxylic acid (Br2–NDI) via Suzuki, Sonogashira and Buchwald–Hartwig coupling reactions with either boronic acid derivatives, alkynes, or amines.
This approach provides access to products with varying electronic properties, as demonstrated by the range of colors of the products in solution (see below). The researchers also synthesized molecules containing two c-NDI residues (pictured above on the right) which have previously been used in the acceptor layer in oPVCs.

The mechanochemical synthesis is greener than conventional processes because it uses no solvent. This removes the energy and environmental costs of solvent production, transportation, and disposal. The reactions are tolerant to oxygen and moisture and are often complete in under 90 min.
- Solvent free synthesis of core‐functionalised naphthalene diimides using a vibratory ball mill: Suzuki, Sonogashira and Buchwald‐Hartwig reactions,
Lydia Panther, Daniel Guest, Andrew McGown, Hugo Emerit, Raysa Tareque, Arathy Jose, Chris M. Dadswell, Simon Coles, Graham Tizzard, Ramon Gonzalez-Mendez, Charles Goodall, Mark Bagley, John Spencer, Barny William Greenland,
Chem. Eur. J. 2022.
https://doi.org/10.1002/chem.202201444