Pyrazoles are nitrogen-containing heterocyclic compounds. Pyrazole units are often present in agrochemicals and pharmaceuticals and have applications in, e.g., coordination chemistry, catalysis, polymers, and chemosensors. Therefore, the development of straightforward reactions for assembling new pyrazole derivatives is an interesting research target.
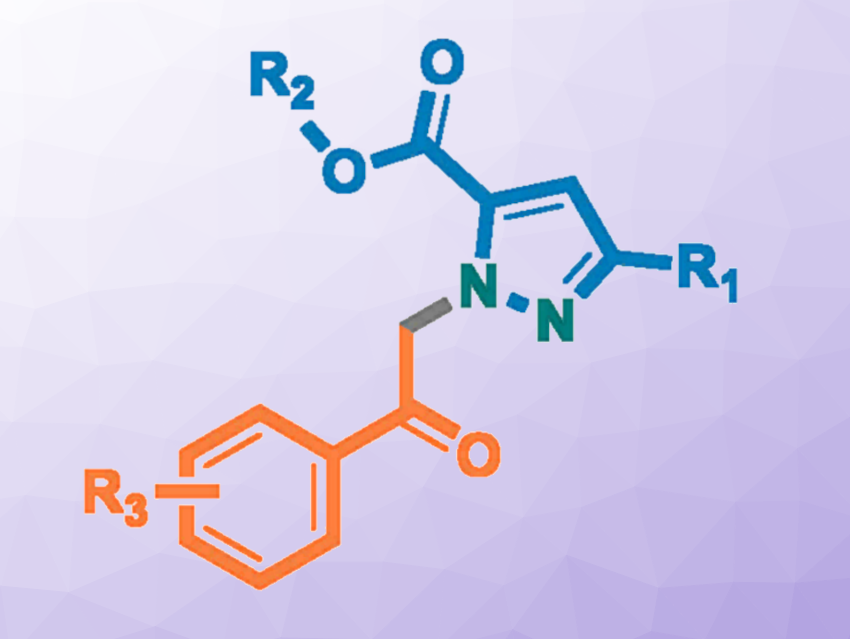
Haifeng Wang, Wuhan Institute of Technology, China, Key Laboratory of Green Chemical Engineering of the Ministry of Education, Wuhan, China, and Hubei Key Laboratory of Novel Reactor and Green Chemical Technology, Wuhan, China, Shuangxi Gu, Wuhan Institute of Technology and Key Laboratory of Green Chemical Engineering of the Ministry of Education, Wuhan, Fener Chen, Wuhan Institute of Technology and Shanghai Engineering Center of Industrial Catalysis for Chiral Drugs, China, and colleagues have developed a green, efficient, and highly regioselective synthesis of pyrazoles (general product structure pictured).
The team used two different diazo compounds as building blocks (resulting parts pictured in blue and orange), together with CF3CH2OTf (2,2,2-trifluoroethyl trifluoromethanesulfonate) as both a solvent and a reaction participant. The reactions were conducted at 70 °C without the need for any additional catalysts.
Under these conditions, the desired pyrazole derivatives were obtained in mostly moderate to good yields. The team successfully synthesized 38 examples of pyrazole derivatives. According to the researchers, the developed method could be a valuable synthetic tool, especially considering the current reliance on metal catalysts.
- Solvent‐Directed Regioselective Cascade Reaction with Diazo Compounds,
Menghua Dong, Chunan Wang, Lewan Li, Yufan Chen, Jie Zeng, Jian Lv, Jie Liao, Haifeng Wang, Shuangxi Gu, Fener Chen,
Eur. J. Org. Chem. 2025.
https://doi.org/10.1002/ejoc.202401336
![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)


