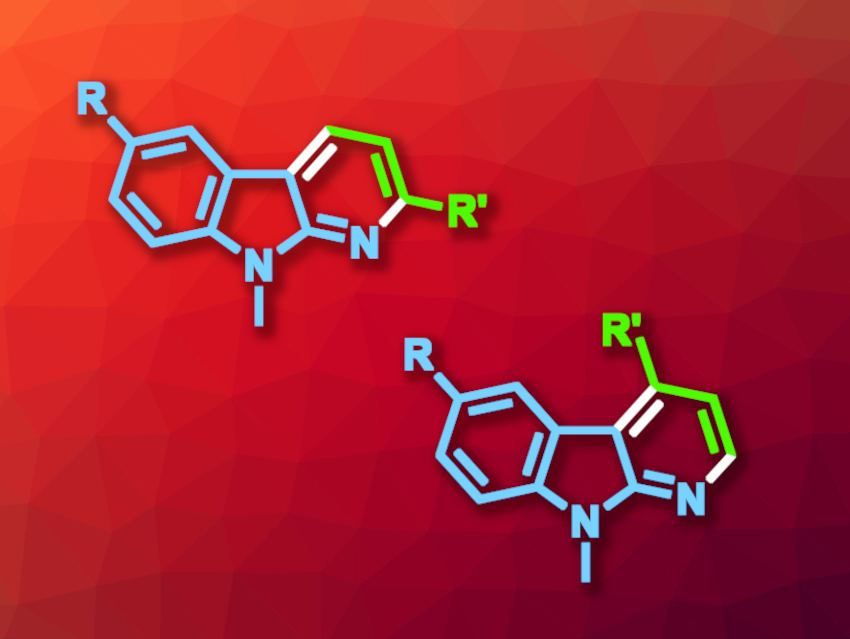
Carbolines can be thought of as an indole fused to a pyridine, and α-carbolines represent one possible isomer of this type of compound. These heterocyclic compounds are often found, e.g., in medicinal chemistry. New synthetic paths to 2- or 4-substituted α-carbolines that are of interest in pharmaceutical chemistry would be useful.
Chinmay Chowdhury, CSIR-Indian Institute of Chemical Biology, Kolkata, India, and colleagues have developed a method for the chemodivergent synthesis of 2- or 4-substituted α-carbolines that can be controlled by the choice of solvent (general product structures pictured above). The team used palladium-catalyzed [3+3] annulations of tosyliminoindolines with α,β-unsaturated aldehydes. They used Pd(bpy)Cl2 as the catalyst and NaOAc as a base. The reactions were performed at 80 °C.
The researchers found that in N,N-dimethylformamide (DMF), the reaction gives 4-substituted α-carbolines, proceeding via a carba-Michael pathway. In N-methylacetamide (NMA), 2-substituted α-carbolines were obtained via an aza-Michael pathway. The reactions gave the desired products in mostly good yields.
In the reaction mechanism proposed by the team, the iminoindoline educt is deprotonated to an amidoindoline, which is palladated at C3 in DMF, followed by a reaction with the α,β-unsaturated aldehyde and ultimately leading to a 4-substituted α-carboline. In NMA, an amino-palladated species is formed from the amidoindoline, ending with a 2-substituted α-carboline. This was supported by the isolation of C3-alkylated products from DMF and N-alkylated products from NMA in control experiments. Overall, the developed approach provides regioselective paths to 2- and 4-substituted α-carbolines from simple substrates.
- A solvent controlled regioselective synthesis of 2- and 4-substituted α-carbolines under palladium catalysis,
Sarat Chatterjee, Rousunara Khatun, Mahammad Ali, Chinmay Chowdhury,
Chem. Commun. 2024.
https://doi.org/10.1039/D4CC00668B