The introduction of unnatural amino acids into a peptide can allow researchers to tune its properties. However, the commercial availability of such amino acids is limited, and their synthesis can require long and tedious processes.
Peter ‘t Hart, Max Planck Institute of Molecular Physiology, Dortmund, Germany, and colleagues have developed a late-stage functionalization method that allows the modification of the standard amino acid lysine after the peptide has been synthesized, directly on a solid support. This method avoids the need for elaborate syntheses of unnatural amino acids.
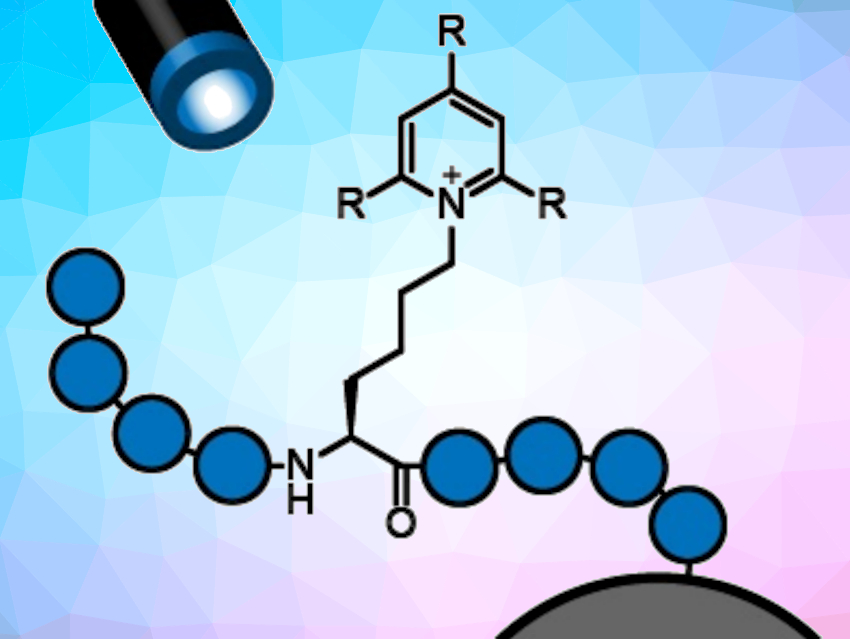
The team first converted the amine groups of lysine side chains to so-called Katritzky salts (pictured below). Katritzky salts are formed from a primary amine and a pyrylium salt and allow for homolytic cleavage of the C–N bond. The salts are cleaved in the presence of Hantzsch ester upon irradiation with blue light. A carbon radical is formed during this process, which can react with various electrophiles to form a new carbon–carbon bond. This then gives a modified amino acid. All reaction steps were performed with the peptide connected to a solid support, which simplifies purification.
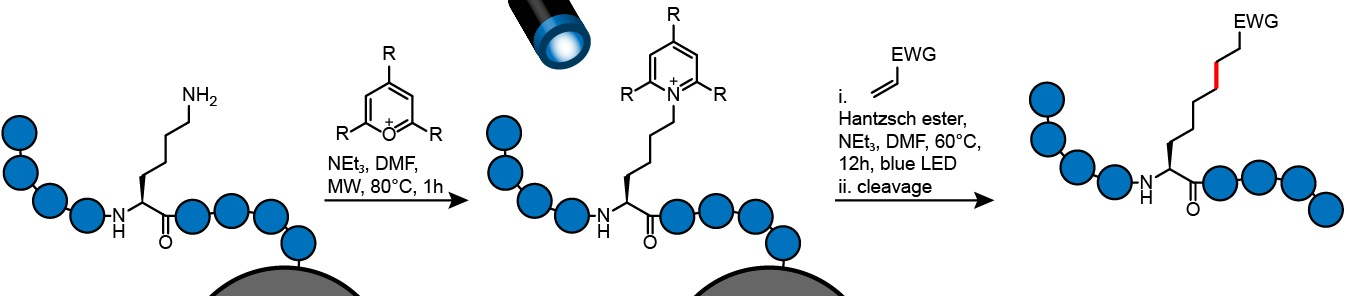
The reaction is compatible with various substrates, lysine analogues, resin supports, and all proteinogenic amino acids. The protocol is easy to perform and allows rapid access to several peptide variants, which can then be screened and their properties optimized.
- Solid‐phase peptide modification via deaminative photochemical Csp3‐Csp3 bond formation using Katritzky salts,
Joseph Openy, Gulshan Amrahova, Jen-Yao Chang, Anaïs Noisier, Peter ‘t Hart,
Chem. Eur. J. 2022.
https://doi.org/10.1002/chem.202201121