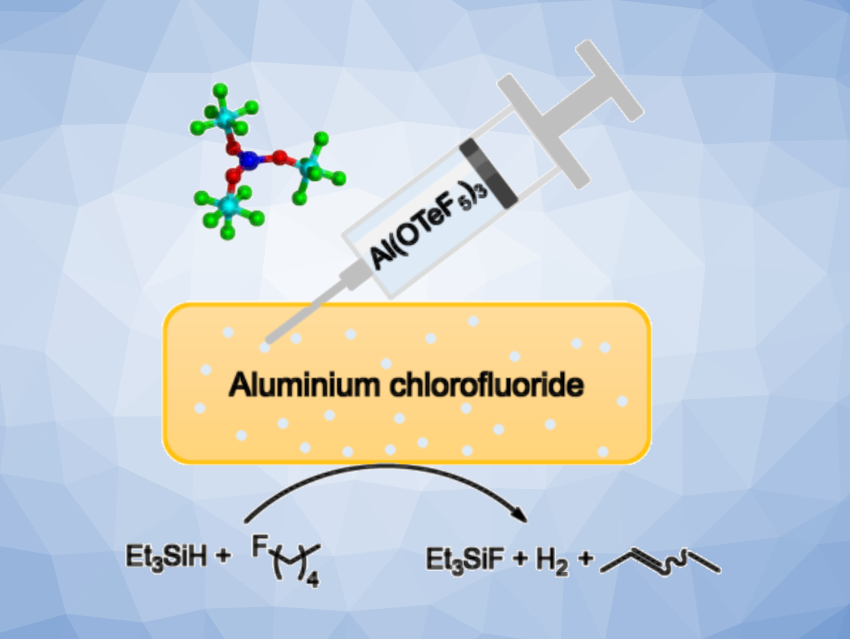
Amorphous aluminium-fluoride-based catalysts can be considered very strong Lewis acids. They are useful, e.g., in conversions such as C–H activation, fluorination, defluorination, or Friedel-Crafts-type reactions. Tuning the properties of aluminium-fluoride-based materials could be useful for the development of improved catalysts.
Thomas Braun, Humboldt University, Berlin, Germany, Sebastian Riedel, Free University Berlin, Germany, and colleagues have developed a route to access a solid Lewis superacid by doping pentafluoroorthotellurate ([−OTeF5], teflate) groups into aluminium chlorofluoride. The teflate group is an interesting substitute for fluoride, because it has similar electron-withdrawing properties, but it is considerably bulkier. The desired doped catalyst (ACF-teflate) was obtained via the fluorination of a mixture of AlCl3 and Al(OTeF5)3 by treatment with excess CFCl3 at –30 °C.
To determine the structure of the catalyst, the team used several analytical techniques, including powder X-ray diffraction (XRD), scanning transmission electron microscopy (STEM), energy-dispersive X-ray (EDX) analysis, and magic-angle spinning (MAS) NMR spectroscopy. The results confirmed the amorphous structure of the product. The sterically demanding [−OTeF5] units cause a pronounced distortion of the bulk material, which induces Lewis superacidity. The team found that the material can catalyze dehydrofluorination reactions of fluoroalkanes to give olefins (example pictured) at room temperature.
- An Amorphous Teflate Doped Aluminium Chlorofluoride: A Solid Lewis‐Superacid for the Dehydrofluorination of Fluoroalkanes,
Minh Bui, Kurt Hoffmann, Thomas Braun, Sebastian Riedel, Christian Heinekamp, Kerstin Scheurell, Gudrun Scholz, Tomasz M. Stawski, Franziska Emmerling,
ChemCatChem 2023.
https://doi.org/10.1002/cctc.202300350