Triplet oxygen atom stabilized by interaction with a Lewis acid under low-temperature matrix-isolation conditions

Beryllium Oxyfluoride Radicals OBeF and OBeF2 Synthesized
![π-Expanded Nanographenes Based on [6]Helicenes](https://www.chemistryviews.org/wp-content/uploads/2024/08/piextendedhelicenenanographene_2024-125x94.png)
π-Expanded Nanographenes Based on [6]Helicenes
Family of chiral nanographenes with [6]helicene units show interesting chiroptical properties
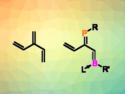
The First Heavy Heterodendralenes
Phosphorus- and boron-containing [3]dendralenes prepared; could serve as precursors to doped polycyclic hydrocarbons
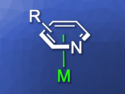
Amination of Aminopyridines via Reversible π Coordination
Ruthenium catalyst acting as an arenophilic π acid in a nucleophilic aromatic substitution
![Using Skipped Enynes in [3+3] Annulations with 4-Hydroxycoumarins](https://www.chemistryviews.org/wp-content/uploads/2024/08/skippedenynesannulation_2024-125x94.png)
Using Skipped Enynes in [3+3] Annulations with 4-Hydroxycoumarins
Transformation under palladium/Brønsted-acid catalysis gives fused heterocycles

Molecular Knots Tied with Different Metal Ions
Knotted ligand synthesized using Cu(II) ions as a template allows the preparation of a knotted zinc(II) complex with coordinatively unsaturated metal centers
![Direct Path to Bioactive Benzo[e]isoindoles](https://www.chemistryviews.org/wp-content/uploads/2024/08/benzoisoindole_2024-125x86.png)
Direct Path to Bioactive Benzo[e]isoindoles
Isoindoles are parts of natural products and pharmaceutical compounds with diverse biological activities
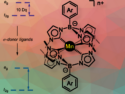
Advanced Manganese Photocatalysts with Boron-NHC Ligands
Can manganese photoredox catalysts enable efficient radical cross-coupling reactions?

Rare Stable Fe(IV)-Superoxo Intermediate at Room Temperature
Presence of a stable Fe(IV)-O₂•⁻ complex inside a nanocage in water for the first time at room temperature reported

Diboratriazole Radical Anion Isolated
One-electron reduction gives π-delocalized radical with an inorganic ring skeleton