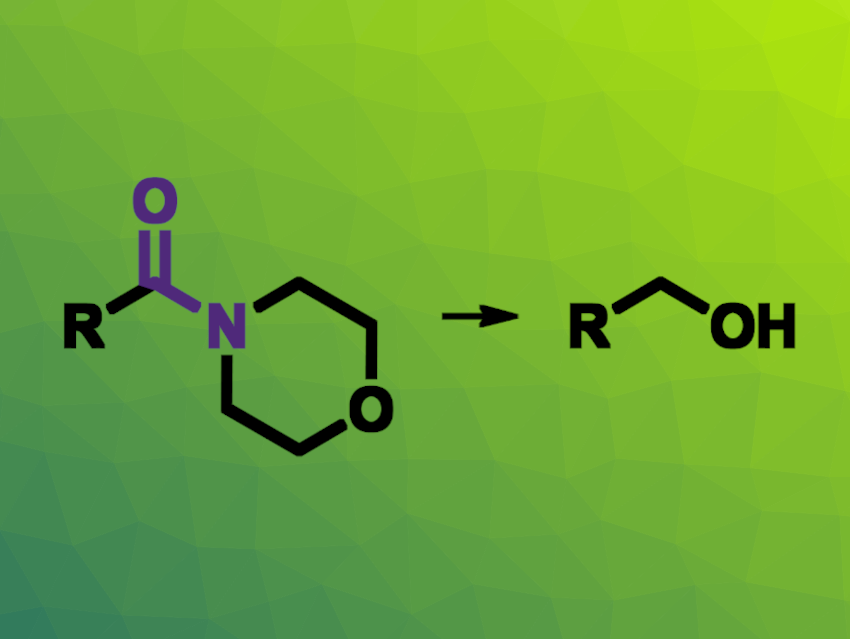
The chemoselective reduction of only one of two amide groups in a molecule can be very challenging to achieve. Usually, the reduction of amides requires harsh reagents such as LiAlH4, which makes it difficult to not reduce all amide groups at the same time. Transformations that use H2 as a less reactive reductant, together with a suitable catalyst, could be promising alternatives to achieve site selectivity.
Johannes F. Teichert, Technical University of Chemnitz, Germany, and colleagues have found that a bifunctional, copper(I)-based catalyst can be used for the site-selective reduction of amides with certain heterocyclic substituents using H2 (example pictured). The catalyst consists of a copper(I) N-heterocyclic carbene (NHC) complex that is covalently linked to a guanidine unit that acts as an organocatalyst. The copper complex forms a hydride in situ with the H2, and the guanidine unit tunes the reactivity of this usually weakly nucleophilic hydride and forms hydrogen bonds with suitable substrates, improving selectivity.
Using this bifunctional catalyst, the team reduced a variety of morpholine-derived amides and obtained the desired alcohols in generally moderate to good yields. Amide groups with other heterocyclic substituents such as thiomorpholine or piperazine were also suitable for this reduction. Overall, the work allows the conversion of usually difficult-to-reduce amides using H2 as the terminal reductant and the site-selective transformations of certain amides based on heterocycles.
- Site-Selective Copper(I)-Catalyzed Hydrogenation of Amides,
Dimitrios-Ioannis Tzaras, Mahadeb Gorai, Thomas Jacquemin, Thiemo Arndt, Birte M. Zimmermann, Martin Breugst, Johannes F. Teichert,
J. Am. Chem. Soc. 2025.
https://doi.org/10.1021/jacs.4c14174