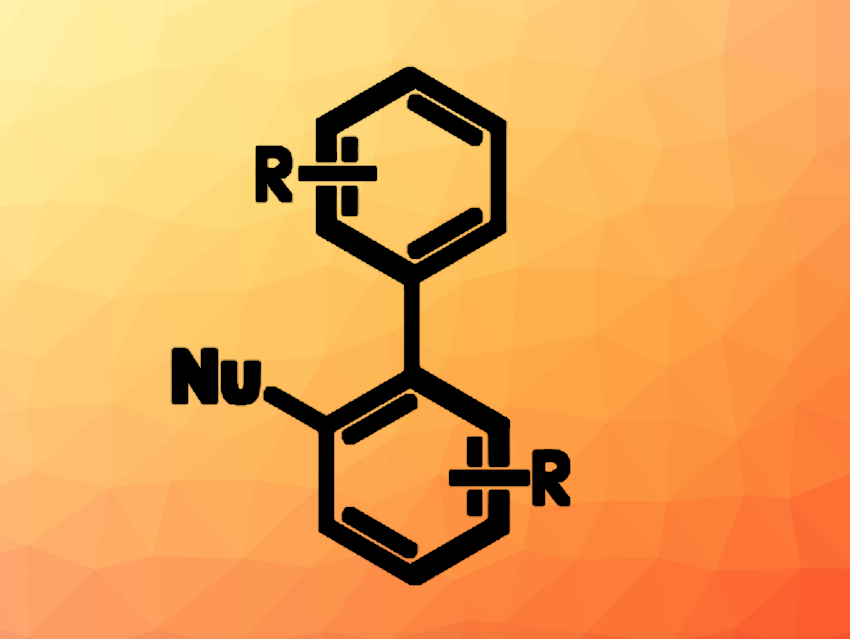
Axially chiral, or atropisomeric, biaryl compounds can be useful, e.g., as ligands in enantioselective catalysis or in drug development. Their chirality is based on the hindered rotation around a σ bond due to the steric hindrance of neighboring groups. Preparing such biaryls from unfunctionalized starting materials via C–H functionalization reactions can be useful, but generally requires directing groups and/or transition-metal catalysts, and existing approaches can have a limited substrate scope.
David A. Nicewicz, University of North Carolina at Chapel Hill, USA, and colleagues have developed a general approach to the coupling of biaryls with a range of nucleophiles to obtain atropisomers using organic photoredox catalysis. The team used an acridinium-based photoredox catalyst (3,6-di-tert-butyl-9-mesityl-10-phenylacridin-10-ium tetrafluoroborate) to react a wide range of biaryl compounds with different nucleophiles, e.g., pyrazole derivatives, nitriles, or amines. The reactions were performed under 427 nm LED light in the presence of (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) as a radical initiator and O2 as an oxidant, using dichloromethane (DCM) as the solvent.
Under these conditions, the desired biaryl atropisomers were obtained in moderate to excellent yields. The method does not require transition-metal catalysts, prior functionalization, or pre-existing directing groups. Overall, the work provides access to a broad range of structurally diverse axially chiral biaryl compounds.
- Synthesis of Biaryl Atropisomers via Site-Selective C–H Functionalization,
Jason C. Genova, David A. Nicewicz,
Org. Lett. 2025.
https://doi.org/10.1021/acs.orglett.5c00121