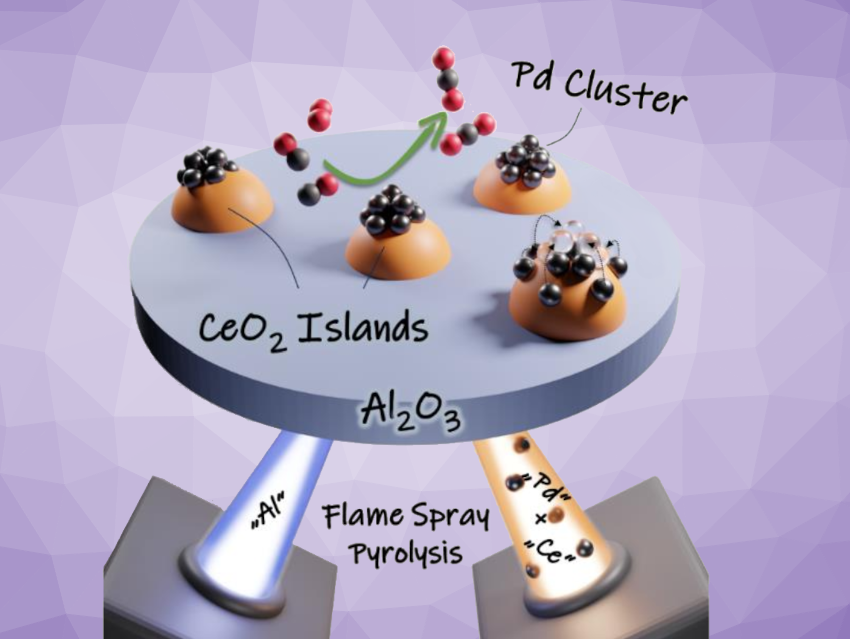
Noble metal clusters or single atoms can be useful catalysts for a variety of reactions. In particular, highly dispersed noble metals supported on ceria (CeO2) can avoid poisoning by CO at low temperatures, because CO can be oxidized at the interface between the noble metal and the ceria. The CeO2 support can also stabilize the dispersed state of the noble metal due to its strong interactions with the metal. However, under the reaction conditions, redispersion can occur and reduce the catalytic activity.
Jan-Dierk Grunwaldt, Karlsruhe Institute of Technology (KIT), Germany, and colleagues have developed a catalyst that is composed of palladium clusters supported on separate, nanosized CeO2 “islands” on an alumina (Al2O3) base (pictured schematically). The catalyst was prepared using a double-nozzle flame spray pyrolysis approach, in which Pd and CeO2 precursors were sprayed in one flame, and the Al2O3 precursor in the other.
The team used palladium acetylacetonate as the Pd precursor, cerium ethylhexanoate as the ceria precursor, and aluminium acetylacetonate as the alumina precursor. The resulting particles were collected on glass-fiber filters and calcined at 500 °C. The formation of small Pd clusters was observed at a rather low noble-metal loading of 0.5 wt.%.
The researchers used the developed catalyst for the oxidation of CO. They found that the confinement of the Pd clusters on the CeO2 island prevents sintering or redispersion during the reaction. This allowed the catalyst to maintain a high CO oxidation activity at low temperatures.
- Highly Active Oxidation Catalysts through Confining Pd Clusters on CeO2 Nano‐Islands,
Jan-Dierk Grunwaldt, Daria Gashnikova, Florian Maurer, Eric Sauter, Sarah Bernart, Jelena Jelic, Paolo Dolcet, Carina B. Maliakkal, Yuemin Wang, Christof Wöll, Felix Studt, Christian Kübel, Maria Casapu,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202408511