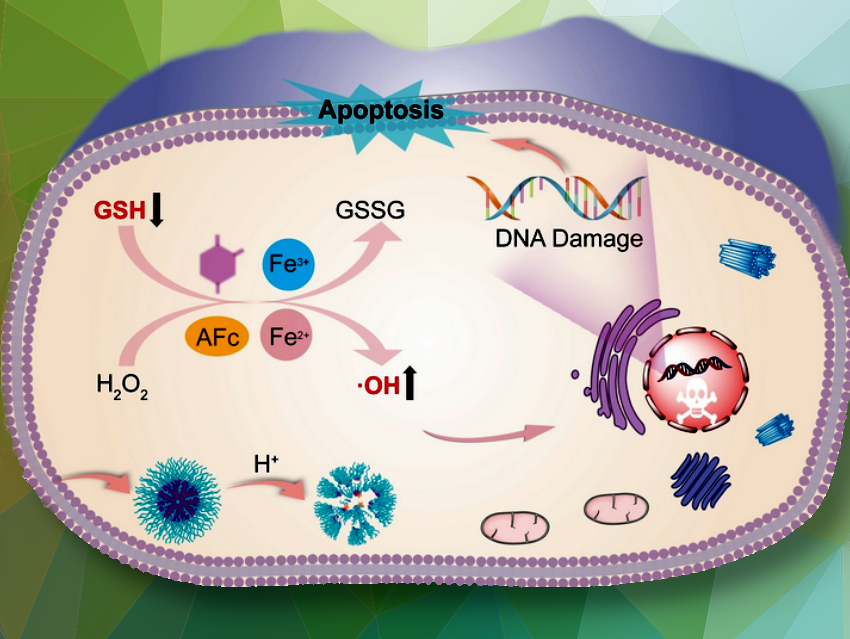
Antitumor agents must kill off cancer cells while protecting healthy tissue and having no toxic side-effects. A novel approach based on “self-immolative” polyferrocenes—copolymers that split apart into their components as soon as they enter a tumor cell—could meet these demands. The drugs they hold then synergistically cause an abrupt increase in free radicals and incapacitate the defenses of tumor cells.
Self-Assembly of Self-Immolative Amphiphilic Poly(ferrocenes), BPnbs-Fc
Xianglong Hu and Shiyong Liu, University of Science and Technology of China, Hefei, China, and colleagues have incorporated two synergistically cooperative molecules together into a copolymer. The copolymer chains are hydrophobic at one end and hydrophilic at the other. In aqueous environments, they aggregate into nanoparticles in which the hydrophobic ends are all pointed toward the inside and the outer, hydrophilic portions, are shielded by polyethylene glycol moieties.
Polyethylene glycol is a nontoxic polymer that is widely used in cosmetics and pharmaceuticals. The polyethylene glycol layer prevents the nanoparticles from being rapidly broken up in the blood by components of the immune system.
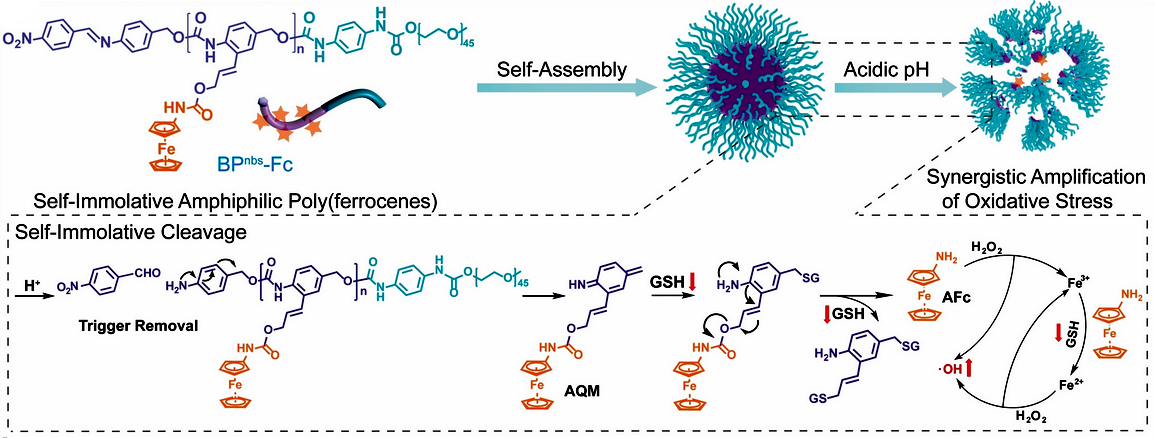
In healthy tissues, the nanoparticles remain intact and inactive. Only the significantly more acidic environment of tumor cells triggers the process of “self-immolation” (pictured above). The particles fall apart and the hydrophobic ends of the polymer chains split apart into their individual building blocks. These are made from azaquinone methide (AQM) units carrying aminoferrocene sidechains.
Application of Drug-Free Amphiphilic Poly(ferrocenes)
Within the tumor cells, glutathione is subsequently activated. Glutathione is a free radical trap and antioxidant that helps to remove foreign substances from cells. Glutathione attacks the azaquinone methide units, binds to them, and splits off the iron sandwiches. Once released, this complex reacts with hydrogen peroxide present in the cells to form hydroxyl radicals (•OH).
In this process the divalent iron in the complex is oxidized to trivalent iron, which the glutathione reduces back to FeII—a fatal cycle that consumes the glutathione and abruptly produces a high concentration of hydroxyl radicals in the cell. The synergy of these two processes puts the tumor cells under severe oxidative stress, which damages and kills them.
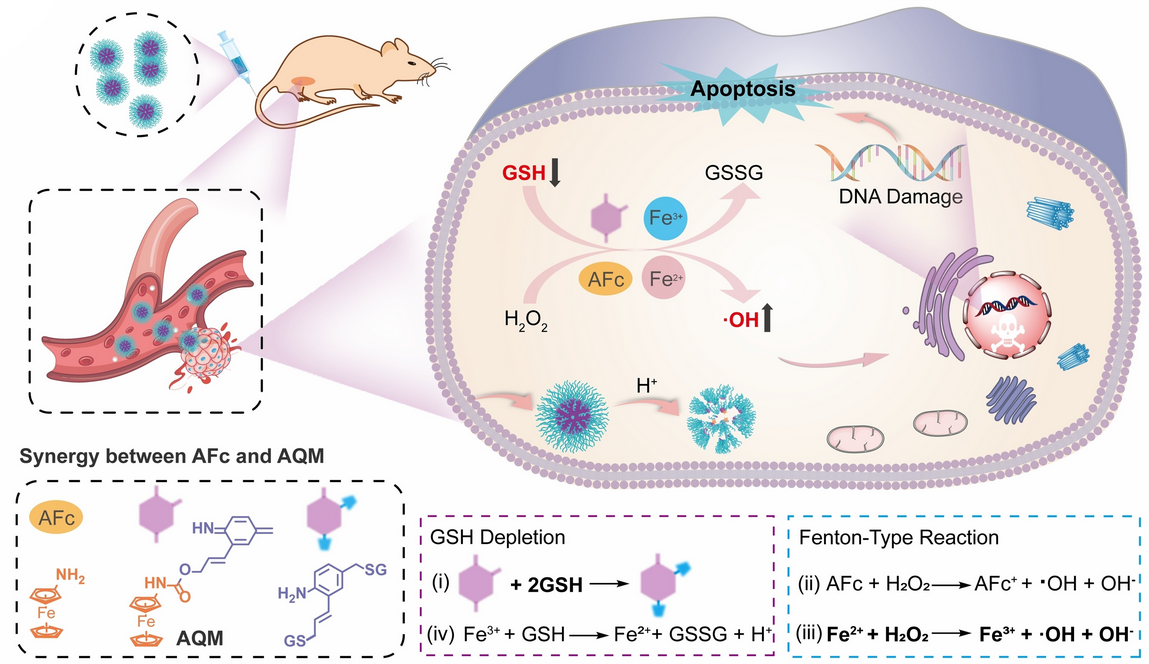
Experiments performed by the team both in vitro and in mice with tumors demonstrated that this efficiently inhibited tumor growth with negligible side-effects. According to the researchers, this approach could allow for new possibilities in the chemodynamic therapy (CDT) of tumors.
Reference
- Self-Immolative Amphiphilic Poly(ferrocenes) for Synergistic Amplification of Oxidative Stress in Tumor Therapy,
,
Angewandte Chemie International Edition 2023.
https://doi.org/10.1002/anie.202303829