Breast cancer (BC) is the most commonly diagnosed cancer worldwide and ranks as the second most fatal disease among women. Although BC is often considered a single disease, it actually comprises three major subtypes of malignant tumors. Approximately 70 % of BC patients are diagnosed with estrogen receptor positive (ER+) and human epidermal growth factor-2 (HER2) negative tumors. These tumors rely on the binding of estrogens to estrogen receptors (ER) present on the surface of the nuclear membrane for their growth and progression. Consequently, the primary treatment for ER+ BC is antiestrogen therapy, also known as endocrine therapy (ET).
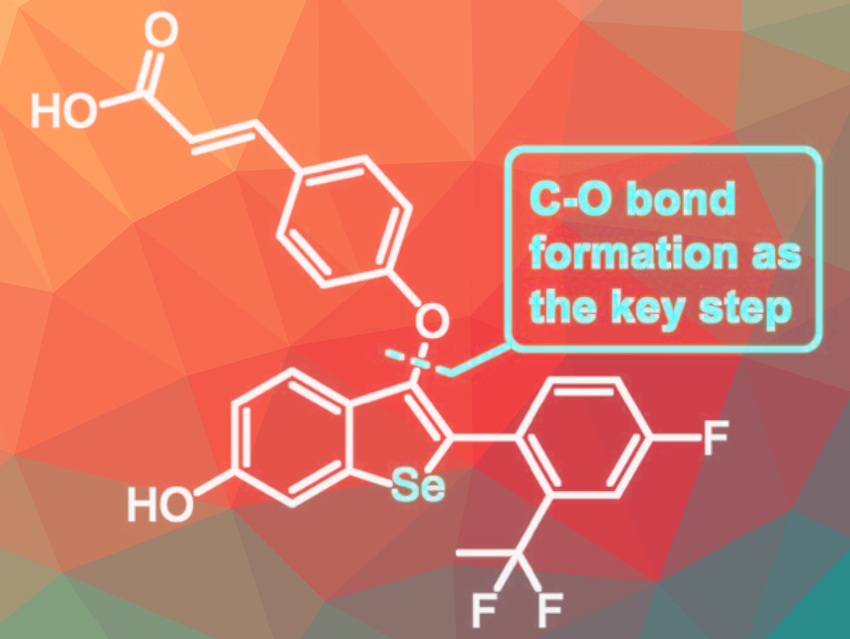
As the initial medical intervention for ER+ breast cancers, selective estrogen receptor modulators (SERMs) and downregulators (SERDs) are used. Researchers Edgars Paegle and Pavel Arsenyan, Latvian Institute of Organic Synthesis, Riga, Latvia, have developed a 10-step synthetic procedure to prepare LSZ102, a selenium analog of a specific SERD agent (depicted in the picture).
The key steps for the synthesis are the cyclization of aryl alkyne intermediate under selenobromination conditions and the subsequent Cu/Fe/O = PPh3 catalytic C–O bond formation.
Biological activity data obtained from the study demonstrated that benzo[b]selenophene serves as a promising heterocyclic core structure for the further development of ER-α modulators and downregulators. The compounds showed strong binding to ER-α, with very low IC50 values (as low as 0.35 nM) and demonstrated moderate to negligible cytotoxic effects on rat cardiomyoblasts. These findings provide additional support for the notion that the synthesis of biologically active compounds with chalcogen (O, S, Se, and potentially Te) analogues can serve as a valuable tool for finetuning the observed biological activity of these compounds.
- Selenium Analogue of LSZ102 as a Highly Potent ER‐α Binder,
Edgars Paegle, Pavel Arsenyan,
European Journal of Organic Chemistry 2023.
https://doi.org/10.1002/ejoc.202300460