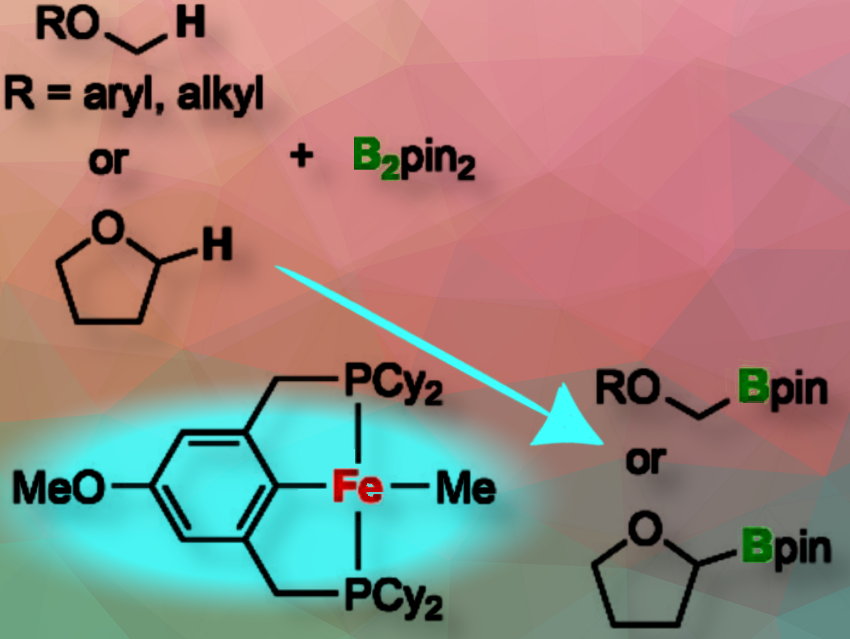
Ken Sakata, Toho University, Chiba, Japan, and Yoshiaki Nishibayashi, The University of Tokyo, Japan, together with colleagues, have synthesized new iron complexes with benzene-based PCP-type pincer ligands and found that these complexes act as efficient catalysts for the highly selective C(sp³)-H borylation of methoxy groups in a range of aryl and alkyl methyl ethers under mild reaction conditions.
A PCP-type pincer is a ligand with two phosphorus atoms and one carbon atom that binds to a metal.
What did you do?
We have achieved the highly selective catalytic C(sp³)−H borylation of methoxy groups in various aryl and alkyl methyl ethers, such as 4-methoxyanisole and 1-methoxybutane, using bis(pinacolato)diboron under mild reaction conditions. This was accomplished with iron complexes bearing benzene-based PCP-type pincer ligands, namely [FeMe(HPCPR)] (HPCPR = 2,6-(R2PCH2)2C6H3, R = Cy or iPr) and [FeMe(MeOPCPCy)] (MeOPCPCy = 4-MeO-2,6-(Cy2PCH2)2C6H2), as catalysts.
In contrast to our previous study, where we used iron complexes with pyrrole-based PNP-type pincer ligands, [FeMe(PNP)] (PNP = 1,3-bis(dicyclohexylphosphinomethyl)-4,5,6,7-tetrahydroisoindol-2-ide), we have designed iron complexes with anionic benzene-based PCP-type pincer ligands in this study. The newly synthesized iron complexes significantly improved catalytic activity.
Additionally, we found that the C(sp³)−H borylation of a methylene group occurred in the reaction with tetrahydrofuran. Based on both experimental results and DFT calculations, we propose a plausible reaction pathway in which iron-boryl and -alkyl complexes act as key intermediates.
Why are you interested in this?
Transition metal-catalyzed, non-directed C−H borylation enables the formation of organoboron compounds from readily available substrates in an atom-economical manner. These reactions introduce a boron moiety into alkane derivatives by activating otherwise inert C(sp³)−H bonds, which can then be transformed into various functional groups, making organoboron compounds useful intermediates in organic synthesis.
What is new and exciting about your study?
Before our present study, there were only two examples of C(sp³)−H borylation of simple substrates by iron complexes via C−H activation. Our present study broadens the substrate scope and provides insights into the reaction mechanisms of the catalytic reaction.
What is the longer-term vision for your research?
Our future research goal is the development of enantioselective C(sp³)−H borylation using optically active iron complexes with chiral ligands. We believe that this achievement may open a new field of asymmetric reactions, an achievement that has not yet been realized.
Thank you very much for these insights.
The paper they talked about:
- Selective Borylation of C(sp3)−H Bond of Methyl Ethers Catalyzed by Iron Complexes Bearing Anionic Benzene-Based PCP-Pincer Ligands,
Shogo Kuriyama, Yuto Suga, Ken Sakata, Yoshiaki Nishibayashi,
ChemistryEurope 2025.
https://doi.org/10.1002/ceur.202400100
Yoshiaki Nishibayashi is a Professor in the Department of Applied Chemistry, School of Engineering, at the University of Tokyo, Japan.
Ken Sakata is a Professor at the Faculty of Pharmaceutical Sciences, Toho University, in Miyama, Funabashi, Chiba, Japan.