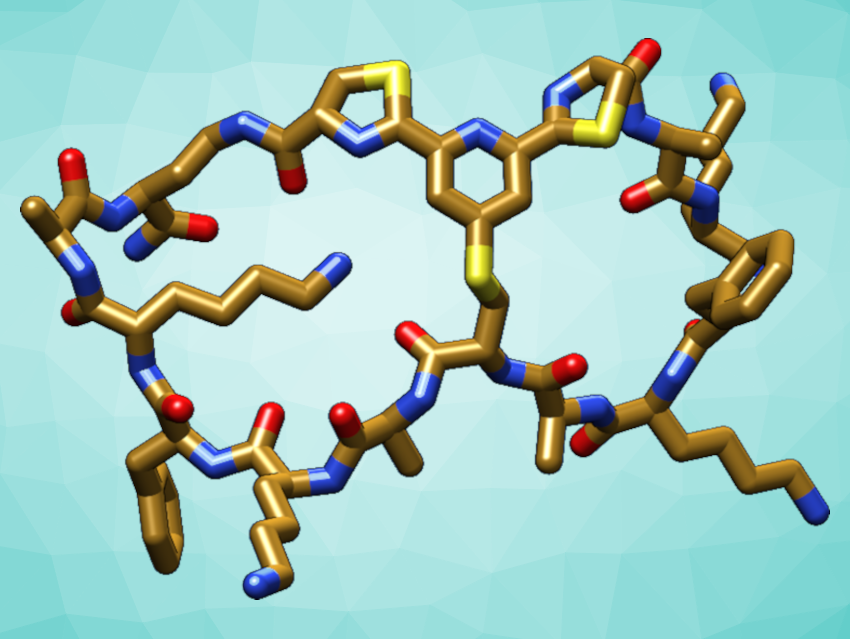
Peptides are short biopolymers made of amino acids. They perform important functions in the human body and can also be used, e.g., in vaccines or drugs. The shapes of peptides play a role in determining their functions. When constrained by cyclization, peptides can gain enhanced stability and bioactivity, which makes them better starting points for drug development. Double cyclization into a “pretzel”-like shape can further improve these properties. However, methods for the chemical synthesis of bicyclic peptides are often tedious and not biocompatible.
Christoph Nitsche and colleagues, Australian National University (ANU), Canberra, have developed a new biocompatible path to bicyclic peptides by linking three functional groups that are connected to the ends and the middle of a peptide chain (pictured below). Both cycles are formed at the same time in a reaction of one 2,6-dicyanopyridine group with two 1,2-aminothiol groups. The required reactive groups can be introduced into the peptide chain during automated synthesis.
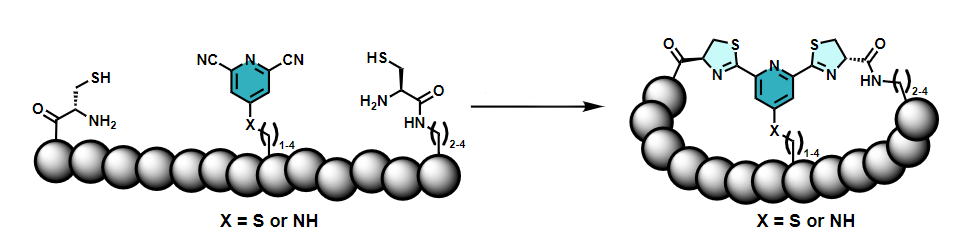
The reaction proceeds in water at physiological pH and does not require organic solvents or catalysts. The researchers showed that their synthetic strategy can be used to stabilize the structure of an antimicrobial peptide (a derivative of the short peptide aurein 1.2). It can also be useful to improve the activity of peptides, e.g., an inhibitor of Zika virus protease, an enzyme that is essential for viral replication.
- Biocompatible and Selective Generation of Bicyclic Peptides,
Sven Ullrich, Josemon George, Alexandra Coram, Richard Morewood, Christoph Nitsche,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202208400