Aromatic amines are useful products and intermediates in organic synthesis. Methods for their synthesis via C–H bond functionalizations are interesting research targets. Existing approaches can have drawbacks such as a need for directing groups, issues with regioselectivity, or limited substrate scopes. Electrochemical methods can be useful for arene C–H aminations, but their utility for nondirected, regioselective variants of this reaction has remained limited.
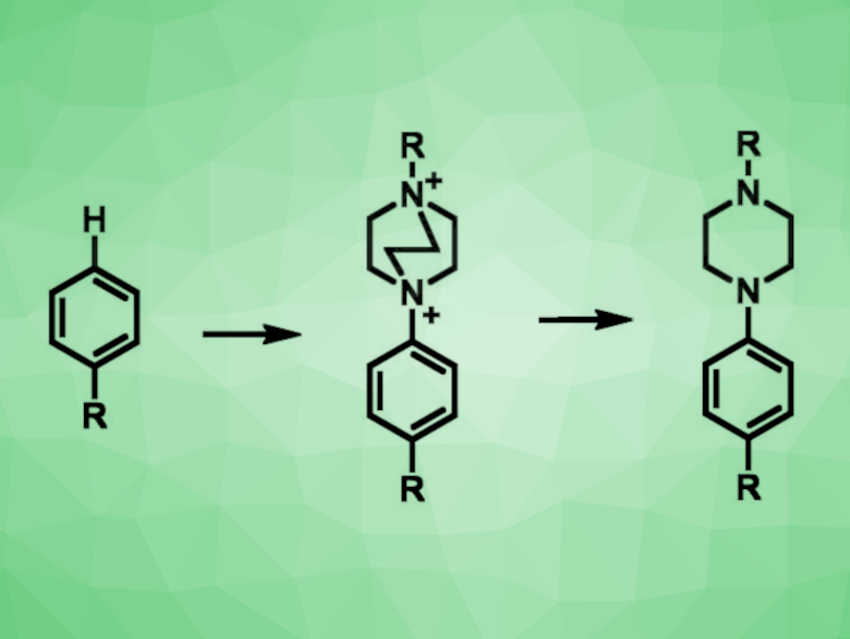
Christian A. Malapit, Northwestern University, Evanston, IL, USA, and colleagues have developed a method for highly selective arene C–H aminations. The team reacted various mono- and disubstituted arenes with Selectfluors I or II, i.e., derivatives of 1,4-diazabicyclo[2.2.2]octane (DABCO) that are usually used as fluorine donors, under electrolysis conditions using MeCN as the solvent and triethylamine as a base. The researchers propose that under these conditions, N-radical dicationic species are formed from the Selectfluor reagent at the cathode. This species can then undergo an N-radical-cation–π interaction with the arene substrate, followed by C–N bond formation and deprotonation.
Using this approach, the team obtained aryl DABCOnium salts (cation pictured above in the center) in moderate to excellent yields and with high regioselectivities for para-C–H functionalization. These products can be further transformed to obtain aryl piperazines (pictured above on the right) or used as aryl radical precursors in photoredox-catalyzed reactions that allow for the formation of C–C or C–P bonds. This shows the potential utility of the developed method for selective arene C–H functionalizations.
- Site-Selective Electrochemical Arene C–H Amination,
Eva Maria Alvarez, Griffin Stewart, Mohammed Ullah, Remy Lalisse, Osvaldo Gutierrez, Christian A. Malapit,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c11506