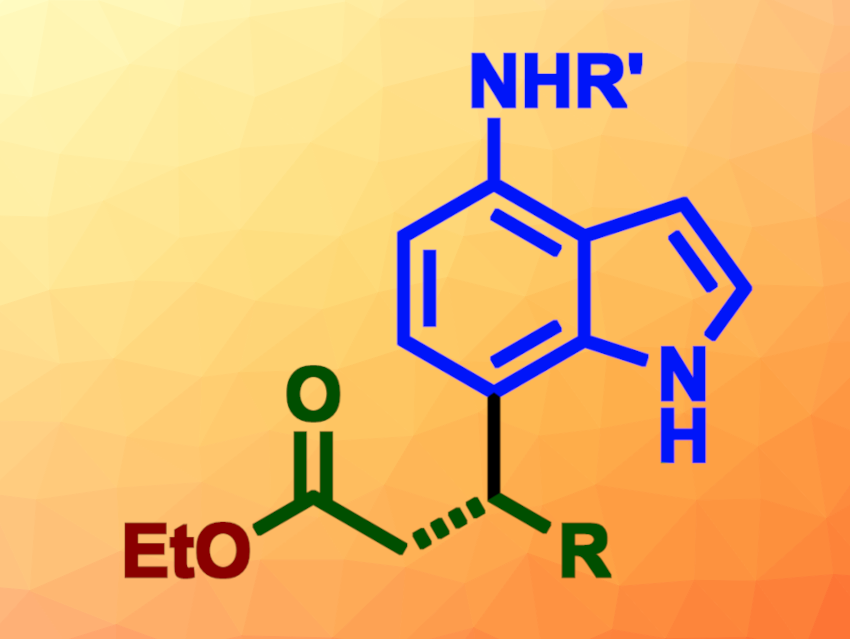
Indoles are heterocyclic compounds that consist of a benzene ring fused to a pyrrole ring. They are important, e.g., in pharmaceutical or agrochemistry. C7-substituted indoles are particularly interesting for drug development. So far, there have only been a few examples of the use of asymmetric organocatalysis to synthesize chiral C7-functionalized indole derivatives.
Tingting Li, Guizhou University, Guiyang, China, and colleagues have developed an N-heterocyclic carbene (NHC)-catalyzed regio- and enantioselective indole C7-alkylation reaction between 4-aminoindoles and α-bromoenals (general product structure pictured). The team reacted different 4-aminoindoles with a variety of α-bromoenals in the presence of alcohols such as ethanol as nucleophiles, using aminoindanol-derived precursors for the NHC catalyst and NaHCO3 as a base. The reactions were performed in CHCl3 under a nitrogen atmosphere at room temperature.
The desired C7-functionalized indoles were obtained in moderate to good yields and with good to excellent enantioselectivities. The team demonstrated the utility of the products by investigating their biological activity against a type of bacteria that can infect Actinidia flowers (a genus of flowers that includes the species that grows kiwis). They found that nine of the products showed promising activities and could, thus, serve as leads for the development of new antibacterial compounds for plant protection.
- N-Heterocyclic Carbene-Catalyzed Regio- and Enantioselective C7-Alkylation of 4-Aminoindoles with α-Bromoenals,
Chenghao Tang, Hui Cai, Chaoyang Song, Xiang Wang, Zhichao Jin, Tingting Li,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.3c04266