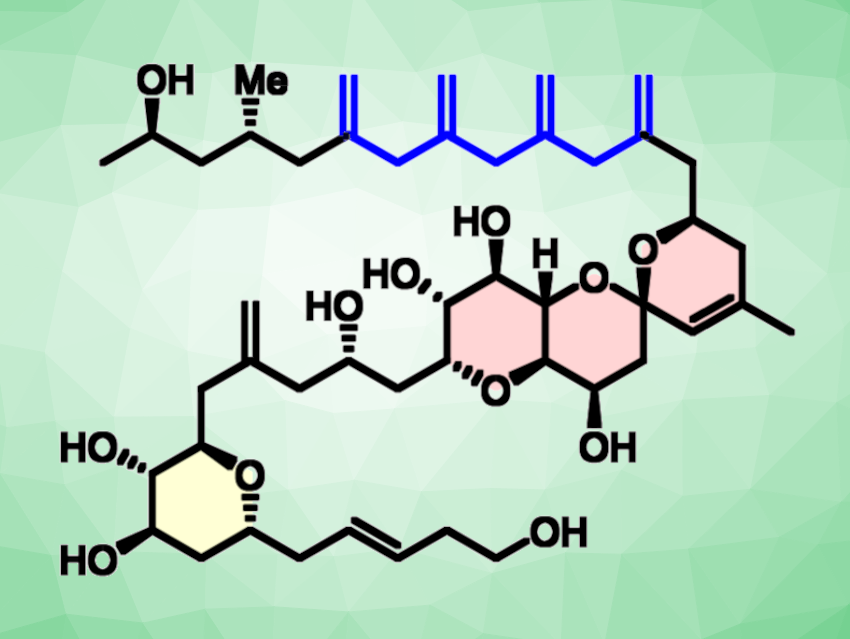
Limaol (pictured) is a natural product derived from a marine dinoflagellate (single-celled organisms). Its five “exo”-methylene groups, with four of them clustered together, make it structurally unique.
Limaol has been synthesized before, but the complex structure posed some challenges, and the developed route turned out not to be very efficient overall (ca. 1.5% yield, 20 steps in the longest linear sequence). It was hampered, e.g., by a low-yielding protecting group cleavage and a long path to one of the molecule’s fragments.
Alois Fürstner, Max Planck Institute for Coal Research, Mülheim an der Ruhr, Germany, and colleagues have improved on their first route to Limaol, developing a more efficient “second generation” total synthesis, with a total of nine steps and an overall yield of 30 %. The team found that the proper choice of protecting groups on the spiroketal core proved to be key for an efficient synthesis. Replacing the previously used bulky silyl ethers with acetates significantly improved the efficiency of the synthesis.
While the first total synthesis gave 3.3 mg of product, the new pathway allowed the team to obtain 277 mg in a single pass. Using the product, the researchers performed a preliminary screening and found that limaol has appreciable antiparasitic activity.
- An Efficient and Scalable “Second Generation” Total Synthesis of the Marine Polyketide Limaol Endowed with Antiparasitic Activity,
Stephan N. Hess, Alois Fürstner,
Chem. Eur. J. 2024.
https://doi.org/10.1002/chem.202401429