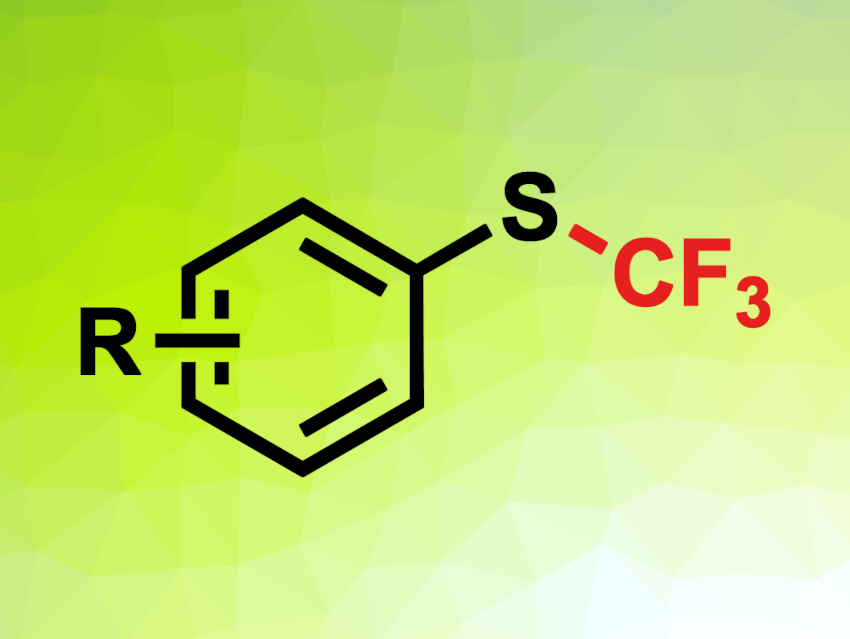
The introduction of fluorine atoms into organic compounds can be useful, e.g., in pharmaceutical chemistry to tune the properties of a drug candidate. Trifluoromethylthio (SCF3) groups, for example, can be used to increase the lipophilicity of a compound. Simple, low-cost methods to introduce this functional group are, thus, interesting.
Aditya Bhattacharyya, Biocon Bristol Myers Squibb R&D Centre, Syngene International Ltd., Bangalore, India, and colleagues have developed a visible-light-mediated trifluoromethylation method for (hetero)aromatic thiols to give the corresponding S-trifluoromethylated derivatives. The team used trifluoromethanesulfonyl chloride (CF3SO2Cl) as an inexpensive precursor for trifluoromethyl radicals. They reacted a variety of thiols with different (hetero)aromatic units (phenyl, benzyl, pyridine, pyrimidine, 1,3,4-thiodiazole, benzo[d]thiazole, or benzo[d]oxazole) with CF3SO2Cl in the presence of the organophotocatalyst 3DPA2FBN (2,4,6-tris(diphenylamino)-3,5-difluorobenzonitrile) under blue LED light (450 nm). The reactions were performed in acetonitrile under a nitrogen atmosphere at room temperature.
Under these conditions, the desired S-trifluoromethylated products were obtained in mostly moderate to high yields. The researchers propose a reaction mechanism that involves the photoexcitation of the organophotocatalyst, followed by a single-electron transfer (SET) from the catalyst to CF3SO2Cl. This reduction results in the formation of a CF3· radical, along with SO2 and a chloride ion. The organophotocatalyst is reduced back to its original form by the thiol, giving a thiol radical cation. Deprotonation and radical–radical coupling then give the product.
- Organic Photoredox-Catalyzed S-Trifluoromethylation of Aromatic and Heteroaromatic Thiols,
Aditya Bhattacharyya, Veeresh Vadde, Manash Pratim Sarmah, M Muthukumar, Arvind Mathur, Richland Tester,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c01818