Transition-metal-catalyzed C–H bond functionalization reactions are important tools in organic synthesis. For example, there has been some research on the carboxylate-directed functionalization of benzoic acids. For the directed C–H functionalizations of acrylic acids, in contrast, there are only a few known examples—in particular, for ruthenium-catalyzed reactions.
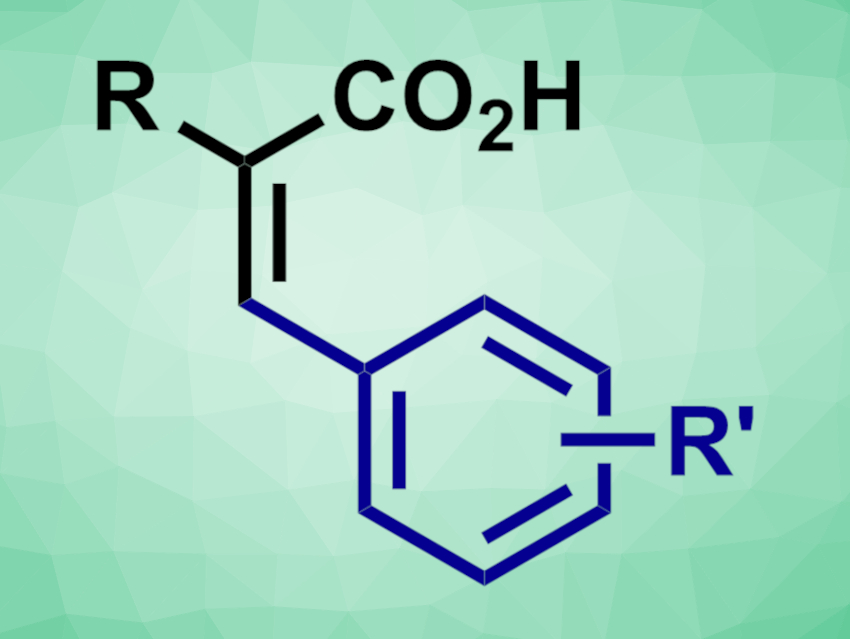
Lukas J. Gooßen, University of Bochum, Germany, and colleagues have developed a ruthenium-catalyzed vinylic C–H arylation of acrylic acids with aryl bromides (general product structure pictured). The team used a catalyst system composed of [Ru(p-cymene)Cl2]2, PEt3·HBF4, and lithium carbonate. N-Methyl-2-pyrrolidone (NMP) was used as the solvent, and the reactions were performed at 100 °C.
The reaction provides selective access to (Z)-diarylacrylic acids. Both electron-rich and electron-deficient aryl bromides underwent the transformation smoothly, and a variety of functional groups were tolerated. The desired products were obtained in moderate to high yields and with high (Z/E)-ratios.
- Ru-Catalyzed C–H Arylation of Acrylic Acids with Aryl Bromides,
Florian Belitz, Ann-Katrin Seitz, Jonas F. Goebel, Zhiyong Hu, Lukas J. Gooßen,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c01043