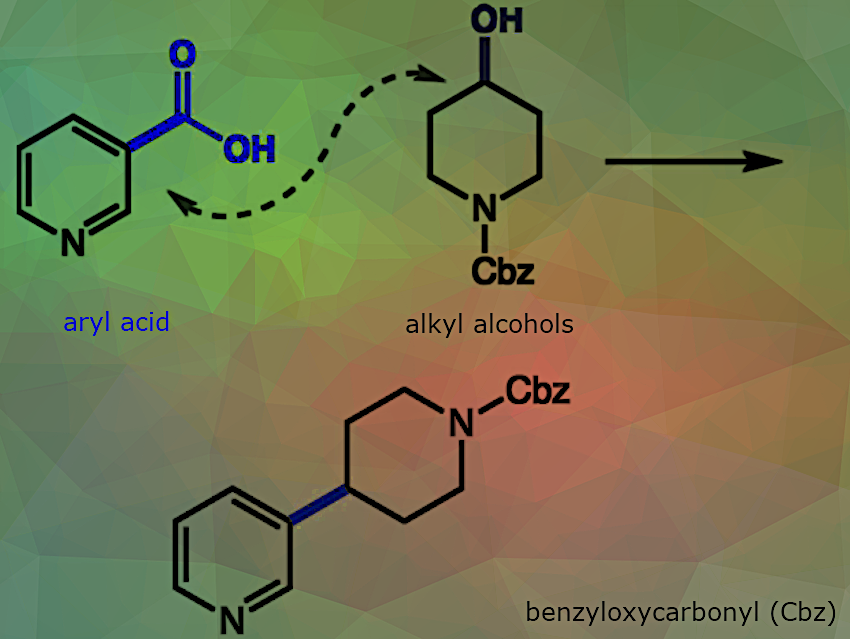
Alcohols and aryl carboxylic acids are among the most commercially available, synthetically versatile, and operationally convenient building blocks in organic chemistry. Despite their widespread availability, the direct formation of C(sp³)–C(sp²) bonds from these functional groups remains a challenge, and existing methods still largely depend on aryl and alkyl halides, which can be expensive, difficult to prepare, or limited in functional group compatibility.
In recent, back-to-back publications [1,2], the Weix and Cernak groups have demonstrated nickel-catalyzed decarbonylation for C–C bond formation, highlighting the potential of aryl carboxylic acids to serve as versatile synthons in cross-coupling reactions. Building on this foundation, Jeffrey R. Long, University of California, Berkeley, and Materials Sciences Division, Lawrence Berkeley National Laboratory, CA, USA, Chi “Chip” Le, Merck & Co., Inc., Boston, MA, USA, David W.C. MacMillan, Merck Center for Catalysis at Princeton University, NJ, USA, and colleagues have described a strategy that combines N-heterocyclic carbene (NHC)-mediated deoxygenation of alcohols with nickel-mediated decarbonylation of aryl acids to form C(sp³)–C(sp²) bonds. This nickel/photoredox-catalyzed approach is applicable to a broad range of aliphatic alcohols and aryl carboxylic acids. The reaction proceeds fully at room temperature and shows excellent functional group tolerance, making it particularly attractive for late-stage functionalization and practical synthetic applications.
First, the aryl carboxylic acid reacts with dipyridin-2-yl carbonate (DPC) and a catalytic amount of 4-dimethylaminopyridine (DMAP) to form an activated acid intermediate in situ. Separately, the alcohol substrate reacts with an NHC salt under mildly basic conditions to generate an NHC–alcohol adduct, also in situ.
Upon visible-light irradiation, the photocatalyst Ir[dF(CF₃)ppy]₂(dtbbpy)PF₆ is excited to a long-lived oxidizing triplet state. This excited state is reductively quenched by the NHC–alcohol adduct via single-electron transfer (SET), forming a reduced Ir(II) species. Deprotonation and β-scission of the resulting intermediate generate an alkyl radical and an inert byproduct.
At the same time, Ni(0) undergoes oxidative addition with the activated acid, followed by decarbonylation to yield an aryl–Ni(II) complex. The alkyl radical is trapped by this Ni(II) complex to form a Ni(III)–alkyl intermediate. Reductive elimination from this intermediate yields the C(sp³)–C(sp²) coupled product and a Ni(I) species. Finally, single-electron transfer from the Ir(II) species to the Ni(I) comlex regenerates both the Ni(0) and Ir(III) catalysts, completing the catalytic cycles.
This combined method significantly broadens the substrate scope, complements existing cross-coupling techniques, and extends the applicability of decarbonylative cross-coupling to new classes of compounds. Notably, the transformation offers an orthogonal strategy to traditional esterification, providing a novel route to complex molecular architectures.
The team used this approach to synthesize a wide array of aryl–alkyl cross-coupled products and modifying complex molecules, including pharmaceuticals, natural products, and biomolecules.
- Aryl Acid-Alcohol Cross-Coupling: C(sp³)–C(sp²) Bond Formation from Nontraditional Precursors,
Eva Lin, Johnny Z. Wang, Edna Mao, Stephanie Tsang, Kurtis M. Carsch, Cesar N. Prieto Kullmer, Ryan E. McNamee, Jeffrey R. Long, Chi “Chip” Le, David W.C. MacMillan,
J. Am. Chem. Soc. 2025.
https://doi.org/10.1021/jacs.4c15827
[1] James L. Douthwaite, Ruheng Zhao, Eunjae Shim, Babak Mahjour, Paul M. Zimmerman,Tim Cernak, Formal Cross-Coupling of Amines and Carboxylic Acids to Form sp³–sp² Carbon–Carbon Bonds, J. Am. Chem. Soc. 2023, 145(20), 10930–10937. https://doi.org/10.1021/jacs.2c11563
- Related ChemistryViews News
Building blocks activated as pyridinium salts and N-acyl-glutarimides for C(sp3)–C(sp2) coupling
[2] Jiang Wang, Lauren E. Ehehalt, Zhidao Huang, Omar M. Beleh, Ilia A. Guzei, Daniel J. Weix, Formation of C(sp²)–C(sp³) Bonds Instead of Amide C–N Bonds from Carboxylic Acid and Amine Substrate Pools by Decarbonylative Cross-Electrophile Coupling, J. Am. Chem. Soc. 2023, 145(18), 9951–9958. https://doi.org/10.1021/jacs.2c11552