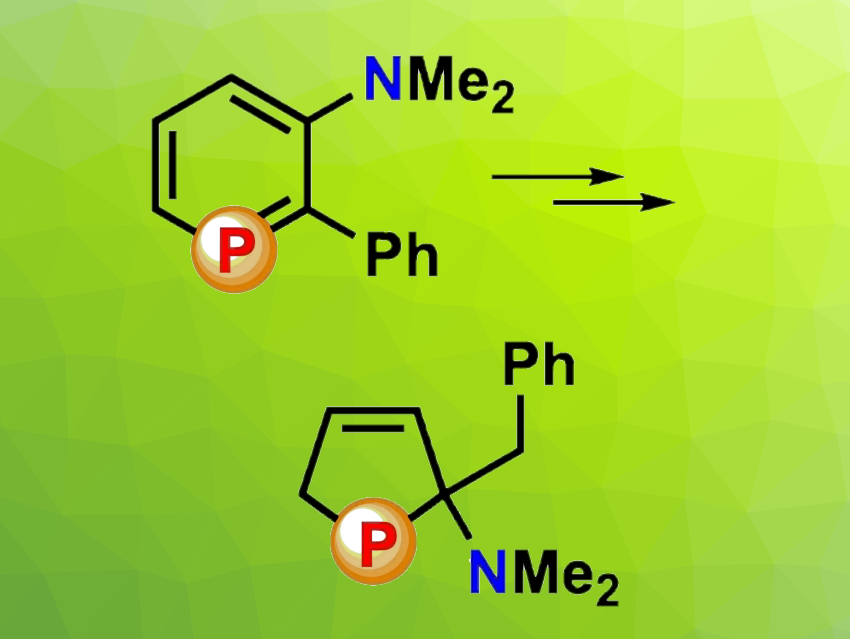
Phosphabenzenes are phosphorus derivatives of arenes and higher homologs of pyridines. They can be useful in fields such as coordination chemistry, bond-activation reactions, (photo)catalysis, and materials science.
Christian Müller, Freie Universität Berlin, Germany, and colleagues have investigated amino-functionalized phosphabenzenes, i.e., phosphorus-containing derivatives of anilines. The team discovered that a 3-amino-functionalized phosphabenzene derivative can undergo a selective and quantitative ring contraction reaction in the presence of hydrochloric acid to form a five-membered hydroxylphospholene oxide, which participates in hydrogen bonding (reaction pictured below, DCM = dichloromethane).
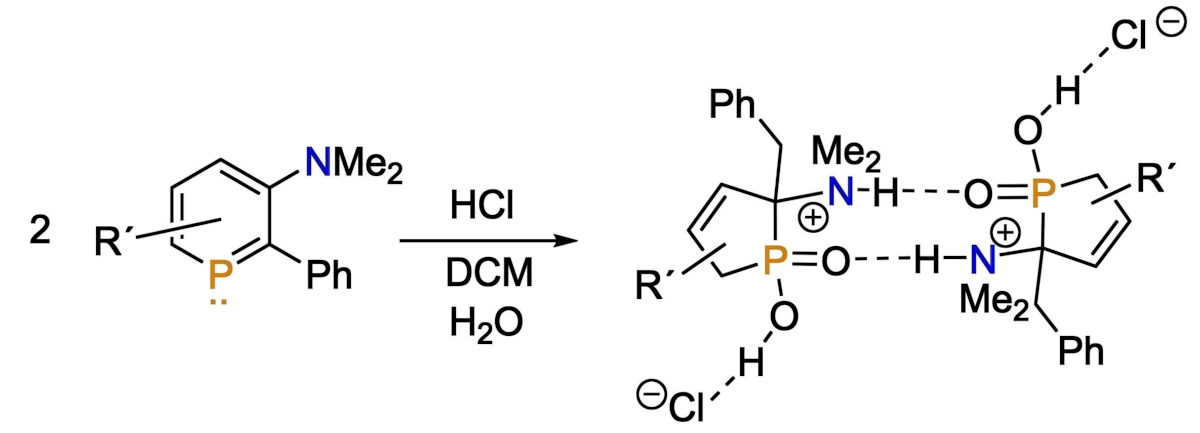
The researchers performed density functional theory (DFT) calculations and deuterium labeling experiments to obtain insight into the reaction mechanism. The team proposes that the reaction starts with the protonation of the amino substituent by HCl and continues with a proton migration, the addition of two molecules of water, and a tautomerization. The rate-determining step then involves the formation of a new P−C bond, followed by a proton transfer to give the product. Overall, in contrast to functionalized pyridines, the special electronic properties of phosphabenzenes allow for interesting reactions at aromatic phosphorus heterocycles.
- Phospholenes from Phosphabenzenes by Selective Ring Contraction,
Jinxiong Lin, Nathan T. Coles, Lea Dettling, Luca Steiner, J. Felix Witte, Beate Paulus, Christian Müller,
Chem. Eur. J. 2022.
https://doi.org/10.1002/chem.202203406