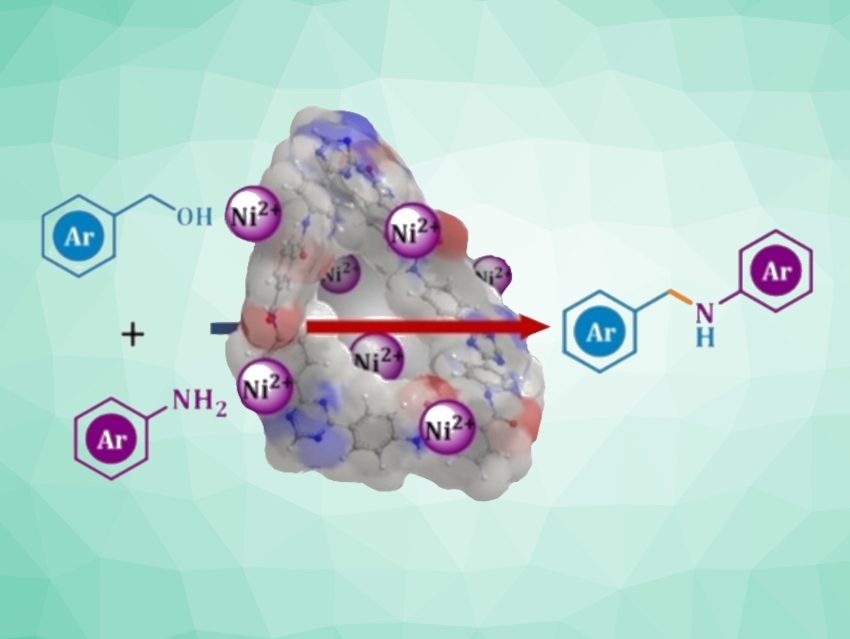
N-Alkylation is a very common type of reaction in organic synthesis. Environmentally friendly and efficient methods for the formation of C–N bonds are, thus, interesting research targets. The direct N-alkylation using alcohols is interesting in this context because it can use renewable, easy-to-obtain feedstocks. This type of reaction can proceed via the conversion of the alcohol to a more reactive carbonyl compound, followed by the condensation with an amine to give an imine intermediate, and completed by a reduction of the C=N unit. This can also be achieved by a catalytic protocol in which a transition metal catalyst “borrows” H2 from the alcohol and transfers it back during the reduction of the imine intermediate. This is known as a borrowing hydrogen strategy.
Bipul Sarma, Tezpur University, Assam, India, and colleagues have developed a reusable catalyst for the N-alkylation of amines via a borrowing hydrogen strategy. The team synthesized a nitrogen-rich porous organic polymer (POP) with embedded Ni(II) catalytic centers for this purpose. They used an amide-functionalized, triazine-based POP that was prepared from 2,4,6-tris-(4-aminophenyl)-1,3,5-triazine (TAPT) and 1,3,5-benzenetricarbonyl chloride (BzCl). The nickel was then incorporated by adding NiCl2·6H2O.
The resulting solid catalyst was used for the N-alkylation of amines using alcohols. The researchers found that the catalyst was suitable for the alkylation of a wide range of anilines/amines with different alcohols. The catalyst system reached turnover numbers (TON) of up to 932 and turnover frequencies (TOF) of up to 51 h−1. The catalyst is easily recovered and can be reused for multiple reaction cycles.
- One‐Pot N‐Alkylation of Amines over Ni(II) Embedded Reusable Porous Organic Polymer via Borrowing Hydrogen Strategy,
Debabrat Pathak, Bikash Kumar Kalita, Himanshu Sharma, Nishant Biswakarma, Ramesh Ch. Deka, Bipul Sarma,
ChemCatChem 2024.
https://doi.org/10.1002/cctc.202301399
Also of Interest
-
What is Borrowing Hydrogen Catalysis?,
ChemistryViews 2016.
A green chemistry approach to activating alcohols