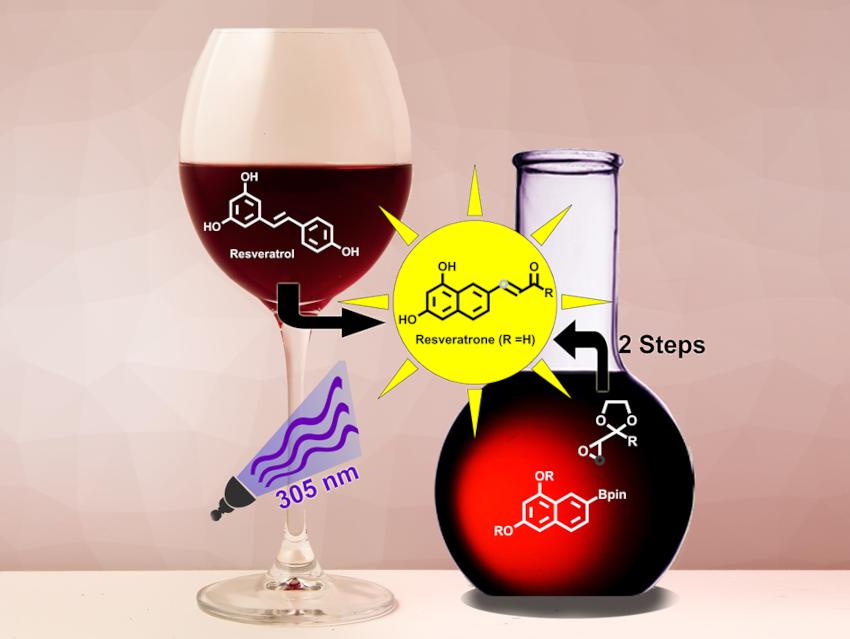
Resveratrone (pictured) is a highly fluorescent molecule. Its photochemical properties make it interesting as a potential fluorescent label for biological applications. Resveratrone was first found after irradiation of trans-resveratrol with UV light. However, obtaining a clean sample by this method and further chemical modifications of resveratrone used to be quite difficult.
Jens Voskuhl, Christoph Hirschhäuser, University of Duisburg-Essen, Essen, Germany, and colleagues have performed the first total synthesis of resveratrone, as well as a regioisomer with comparable photophysical properties, iso-resveratrone. The team first prepared a boronate precursor and an epoxide precursor (pictured below in orange). The boronate building block was prepared from 1,3-dinaphthol, which was protected using tert-butyldimethylsilyl (TBS) groups, followed by a Hartwig-Miyaura borylation. This reaction gives a mixture of the necessary precursors for resveratrone and iso‐resveratrone. The epoxide component was synthesized starting from 3-chlorobutan-2-one.
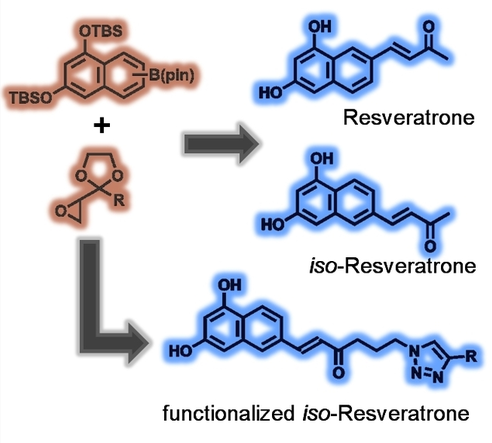
Using the two prepared building blocks, the key reaction in the syntheses is an epoxide olefination using the pinacol boronates. Formerly a twofold excess of boronate was necessary to obtain good yields in this reaction. The excess could be reduced to 1.3 equivalents for aromatic boronates. This is a vital improvement for reactions that require the coupling of valuable boronic esters, such as in this case.
The synthetic route allows for convenient modification, which the team demonstrated by the preparation of an azide derivative that is useful for further transformations via click reactions (example product pictured above).
- Total Synthesis of Resveratrone and iso‐Resveratrone,
Stefan Fritsch, Nazli Aldemir, Jan Balszuweit, Kevin Bojaryn, Jens Voskuhl, Christoph Hirschhäuser,
ChemistryOpen 2022.
https://doi.org/10.1002/open.202200098
Also of Interest
- Video Abstract: Total Synthesis of Resveratrone and Derivatives