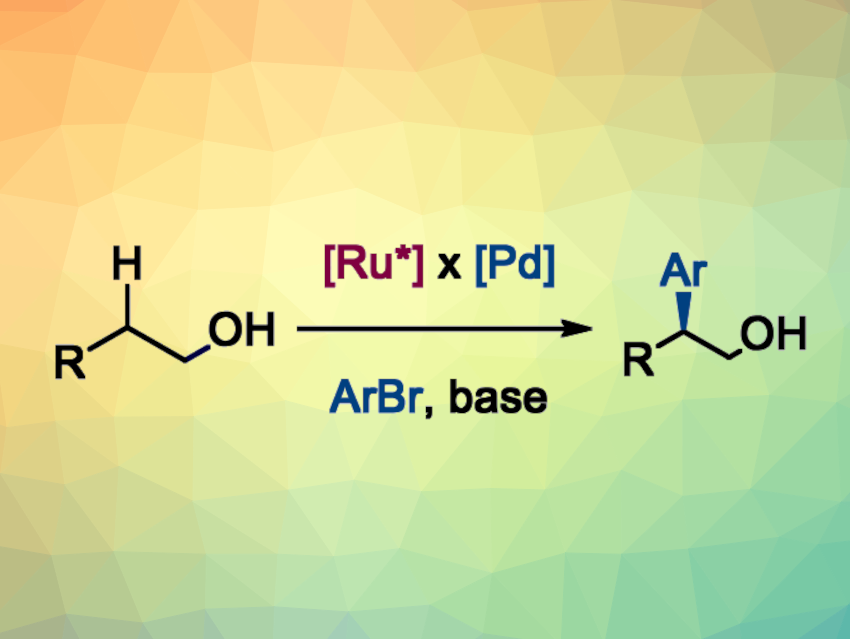
Relay catalysis can be useful to enable otherwise challenging transformations in organic synthesis by combining multiple sequential catalytic reactions. Dynamic kinetic resolution is a strategy that allows the conversion of racemic starting materials into enantioenriched products by exploiting a fast racemization of the educts. The two enantiomers react with different reaction rates in a transformation involving a chiral catalyst or reagent, ultimately leading to the formation of one favored stereoisomer.
Pawel Dydio, University of Cambridge, UK, and University of Strasbourg, CNRS, France, and colleagues have combined the concepts of relay catalysis and dynamic kinetic resolution to enable the regio- and enantioselective C(sp3)−H bond arylation of aliphatic alcohols (general reaction pictured). The team used a chiral, Ru-based catalyst for the reversible oxidation of an alcohol to the corresponding aldehyde. The aldehyde intermediate was arylated using a Pd complex with a bisphosphine ligand. The resulting product can undergo racemization easily, but the Ru-based catalyst controls the stereochemistry of the product by selectively reducing just one of the enantiomers back to an alcohol.
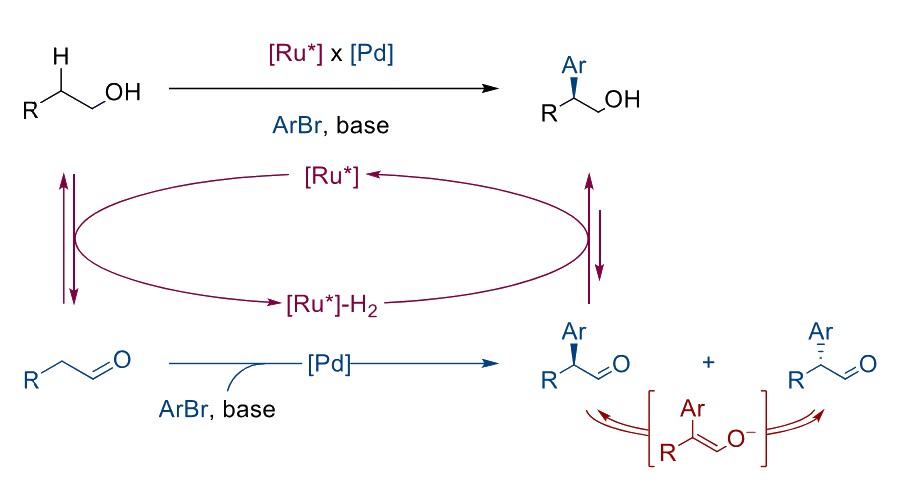
The desired enantioenriched β-aryl alcohols were obtained with enantiomeric ratios up to 98:2 and in yields of 36–74 %. The reactions proceed under mild conditions and use readily available aryl bromides as well as commercially available Ru and Pd complexes.
- A Merger of Relay Catalysis with Dynamic Kinetic Resolution Enables Enantioselective β‐C(sp3)‐H Arylation of Alcohols,
Bruno Lainer, Shuailong Li, Flora Mammadova, Pawel Dydio,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202408418