Heteroarenes substituted with aryl groups are found, e.g., in bioactive molecules and agrochemicals. Arylated pyrrolo[2,3-d]pyrimidine derivatives, for example, can be useful in drug development. Transition-metal-catalyzed direct C–H arylations of heteroarenes can be employed to synthesize this type of heterobiaryl compound.
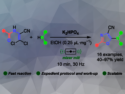
Ting Tang, Hangzhou Normal University, China, Xingxian Zhang, Zhejiang University of Technology, Hangzhou, China, and colleagues have developed a method for the regioselective C6 arylation of pyrrolo[2,3-d]pyrimidines with arylboronic acids (example product pictured). The team used Pd(OAc)2 as the catalyst, TEMPO (i.e., (2,2,6,6-tetramethylpiperidin-1-yl)oxyl) as an oxidant, and CF3CO2H as the solvent. The reactions were performed at room temperature under an air atmosphere.
Under these conditions, a variety of pyrrolo[2,3-d]pyrimidines were reacted with a range of arylboronic acids. The desired arylated products were obtained in moderate to good yields. The team proposes a reaction mechanism that involves the formation of Pd(CF3CO2)2, which reacts with the arylboronic acid. The resulting intermediate reacts with the pyrrolo[2,3-d]pyrimidine in position C5, followed by a 1,2-migration and deprotonation. Reductive elimination then releases the desired product. The resulting Pd(0) species undergoes an oxidative process promoted by TEMPO to regenerate Pd(CF3CO2)2.
- Palladium-Catalyzed Highly Regioselective C6 Arylation of Pyrrolo[2,3-d]pyrimidine Derivatives with Arylboronic Acids,
Min Liu, Chunwei Shen, Ting Tang, Chenhong Pan, Mingrui Liu, Xingxian Zhang,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c00680
![Regioselective Arylation of Pyrrolo[2,3-d]pyrimidines](https://www.chemistryviews.org/wp-content/uploads/2023/04/pyrrolopyrimidines.png)