Pyridine units are very common in organic compounds and are often found, e.g., in natural products, pharmaceutically active compounds, agrochemicals, or materials. Methods for the selective functionalization of pyridine derivatives are, thus, useful tools in organic synthesis. Alkylations, for example, can be achieved by nucleophilic aromatic substitutions, transition-metal-catalyzed reactions, or radical-mediated transformations. However, existing methods can have drawbacks such as limited substrate scopes, a need for optimization, or expensive catalysts. One open challenge is the regiocontrolled alkylation of pyridines at the C4 and C2 positions using a single alkyl source under transition-metal-free conditions.
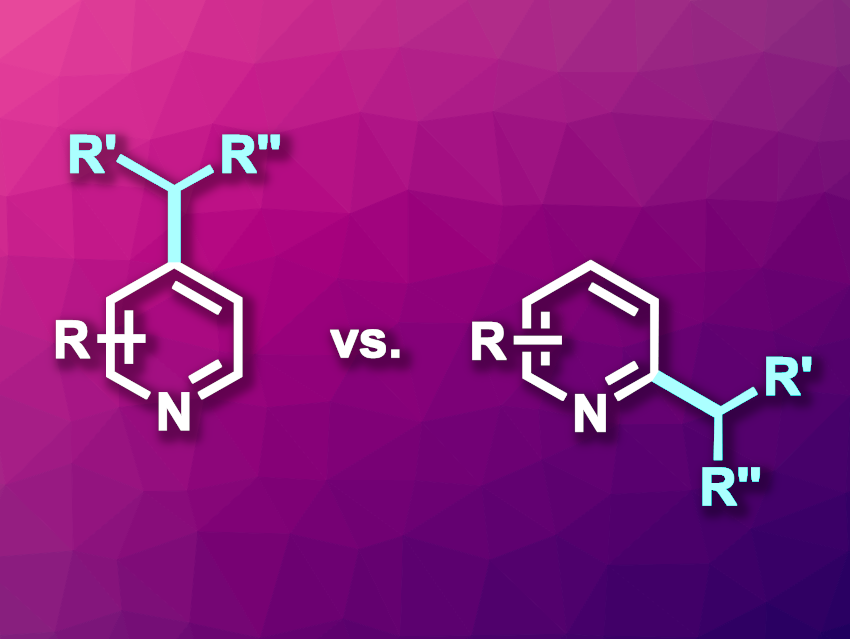
Mu-Hyun Baik, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Republic of Korea, Institute for Basic Science (IBS), Daejeon, Republic of Korea, Seung Hwan Cho, Pohang University of Science and Technology (POSTECH), Republic of Korea, and colleagues have developed a method for just such a regiodivergent alkylations of pyridine derivatives. The team used 1,1-diborylalkanes as the alkylating agent, and the regioselectivity is determined by the alkyllithium activator used with it. Methyllithium as an activator leads to preferred alkylation at the C4 position (pictured above on the left), and sec-butyllithium leads to preferred alkylation at the C2 position (pictured above on the right).
The researchers reacted different pyridine derivatives with a range of 1,1-diborylalkanes, either in the presence of methyllithium in dimethoxyethane (1,2-DME) at 80 °C or in the presence of sec-butyllithium in a tetrahydrofuran (THF)/toluene mixture, also at 80 °C. With methyllithium, they obtained the desired C4-alkylated products in moderate to good yields, and the same was true for the C2-alkylated products obtained when using sec-butyllithium. The method proved suitable for late-stage functionalizations.
Based on mechanistic investigations, the team proposes that the regioselectivity is controlled by the structural dynamics of alkyllithium clusters formed from the activator: Methyllithium forms tetramers that promote C4 alkylations in complexes with molecules of the substrate, and sec-butyllithium forms dimers that similarly promote C2 alkylations. Overall, the work provides a path for previously challenging regiodivergent alkylations of pyridines and demonstrates how alkylithium aggregation states can control regioselectivity.
- Regiodivergent Alkylation of Pyridines: Alkyllithium Clusters Direct Chemical Reactivity,
Woohyun Jo, Chattawat Thangsrikeattigun, Changsu Ryu, Seungcheol Han, Changjin Oh, Mu-Hyun Baik, Seung Hwan Cho,
J. Am. Chem. Soc. 2025.
https://doi.org/10.1021/jacs.4c17198


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
