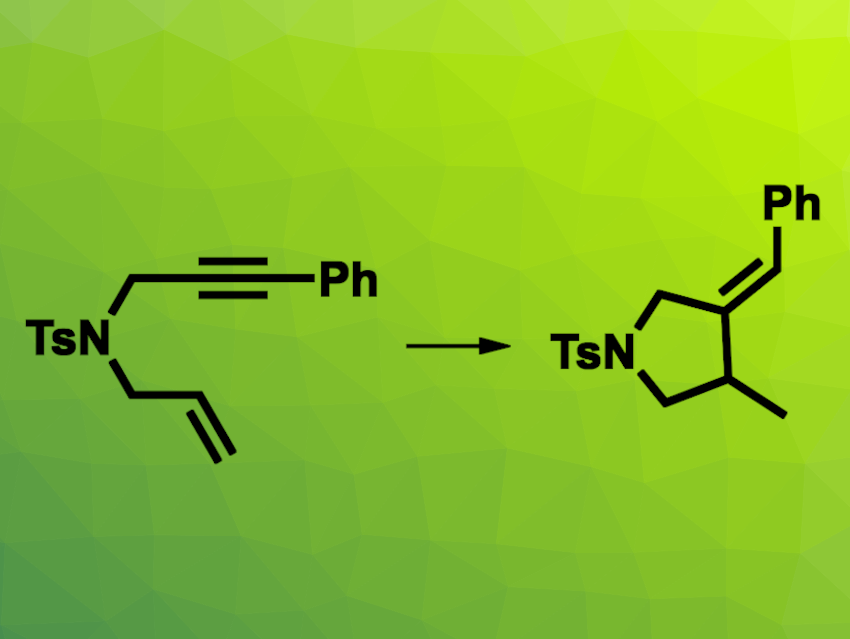
Reductive cyclizations involving two alkyne groups, or an alkyne and an alkene, can be useful for the regio- and stereoselective synthesis of cyclic compounds. Generally, an excess of reductant is required for this type of reaction, which leads to waste. Hydrogen gas could be a useful alternative reductant that is cost-effective and does not produce waste.
Kaho Isoda and Yoshihiro Sato, Hokkaido University, Sapporo, Japan, have developed a cobalt(I)-catalyzed reductive cyclization of diynes and enynes using H2 as a reductant (example reaction pictured). The team used CoBr2·6H2O as the catalyst together with Xyl-Xantphos as a ligand in the presence of zinc and NaBArF4 (sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate. The reactions were performed under 1 atm H2 in dichloroethane (DCE) at 60 °C.
The desired products were obtained in moderate to excellent yields. The reaction tolerates various substituents, including electron-donating and electron-withdrawing groups, as well as oxygen in the chain. The transformation was also successful for an allenyne. The team confirmed that hydrogen gas acts as a reductant in the reaction using control experiments with D2 instead of H2.
- Cobalt(I)-Catalyzed Reductive Cyclization of Enynes and Diynes Using Hydrogen Gas as a Reductant,
Kaho Isoda, Yoshihiro Sato,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c00524