Alessandra Puglisi, Università degli Studi di Milano, Italy, and colleagues have used raw mixtures of rare earth elements (REE) recovered from e-waste as catalysts to promote the stereoselective synthesis of highly valuable compounds.
What is the main idea of your study?
The general idea of the research is to take electronic waste, such as exhaust lamps or magnets, and transform this raw material, which contains rare earth elements (REE), into a catalytically active mixture.
Why are you doing this?
Given their importance for high-tech industries and the concentration of world supply, REEs are of utmost importance for the EU economy and are listed among others as critical raw materials. REE became a critical commodity also because of their growing demand and their small and opaque market. On the other hand, e-waste management has become one of the most critical areas for waste management policies.
The possibility of REE recovery from magnets or fluorescent lamps opens a very interesting opportunity, since REE coming from e-waste represents a large amount of these metals used in terms of economic value.
What is new and cool about your work?
You can take a raw mixture of REE and, with a simple treatment using excess triflic acid, turn it into a catalyst that can promote (stereo)selective organic reactions.
Can you be a bit more specific, please?
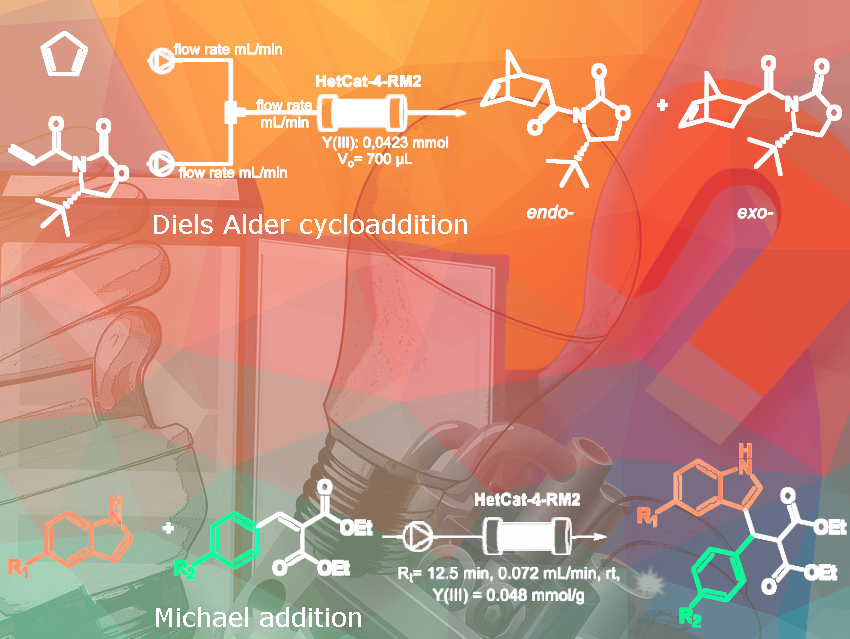
Raw mixtures of REE recovered from e-waste primarily contain Y2O3, which is typically not catalytically active. However, through a simple treatment with excess triflic acid, based on a known protocol, it is converted into Y(OTf)₃, a widely used catalyst that can promote different organic reactions like, among others, Michael addition and Diels-Alder cycloaddition.
In which reactions did you test the catalytic performance?
The raw mixture treated with triflic acid performed very well in the Michael reaction between functionalized indoles and substituted benzylidene diethyl malonates in diethyl ether as solvent in a reaction time of 6 hours, affording from good to excellent yields (up to 91%). It has also been used in a more challenging reaction, the stereoselective Diels-Alder cycloaddition between cyclopentadiene and 4-(S)-3-acryloyl-4-tert-butyl 2-oxazolidinone, at -20°C for 5 hours in dichloromethane, to afford the desired product as a about 80:20 mixture of the endo and the exo isomers. After removal of the chiral auxiliary, the ee was determined to be 91% of the major endo isomer.
Another key finding of this work is that the raw mixture, after treatment with triflic acid, can be immobilized onto functionalized silica through acid-base and electrostatic interactions. This material can promote the Michael addition with the same efficiency as the non-supported mixture, and can be easily recovered and recycled up to 4 times with a slight erosion of the catalytic efficiency. Notably, the supported catalyst can be confined into a flow reactor and work continuously for 4 days. The flow reaction not only allows for simplified recovery of the desired products, but also greatly improves batch productivity.
What effect does the inconsistent mixture of metals in e-waste have on the performance of the catalyst?
We performed some preliminary tests to assess the feasibility of our model reactions in the presence of different metals and we concluded that the presence of other metals in the raw mixture (such as samarium, cobalt, zinc, erbium, aluminum, …) had little or no effect on the catalytic efficiency of the most abundant yttrium.
An interesting point was that the immobilization method was quite selective for yttrium: we noticed, by TEM-EDX analysis of the supported raw mixture after treatment with triflic acid, that yttrium was the only metal detected on the silica surface, together with tungsten, used for the support. This means that, with our immobilization procedure (treatment of the raw mixture with excess triflic acid and immobilization onto silica functionalized with amino group and phosphotungstic acid), we are somehow able to “clean” the raw mixture coming from e-waste and make it an even more efficient catalyst. This finding will be further investigated in more detail in the near future.
What is the aim of your study?
The aim of this study is to demonstrate that raw mixtures of REE recovered from e-waste can be used as catalysts for the synthesis of highly valuable compounds. This approach offers an innovative way to recycle REE, by avoiding the expensive steps necessary to reuse REE in the electronics industry, and instead repurposing them for the synthesis of highly functionalized (chiral) molecules, including biologically active compounds.
What was the most challenging part of your work?
Dealing with complex mixtures of inorganic compounds and trying to highlight the catalytic activity of just one element, yttrium, was definitely challenging. However, the multidisciplinary nature of the team was helpful in overcoming the difficulties we encountered, for example, in determining the composition of the raw mixtures, in handling the raw mixtures, and in the process of preparing and characterizing the supported materials.
What inspired this project?
This work resulted from a very productive collaboration between the University of Milan, the University of Pavia in Italy, and the Institute of Chemical Technology in Freiberg, Germany, as part of the project ‘Innovative Recycling of Rare Earth Elements from Electronic Waste,’ financed by the Cariplo Foundation and the Italian Ministry of Ecological Transition (MITE).
Thank you for these insights.
The paper they talked about:
- Recycling of Rare Earth Elements: From E-Waste to Stereoselective Catalytic Reactions,
Emanuela Donato, Fabrizio Medici, Valerio Chiroli, Alessandra Puglisi, Clemens Rogoli, Peter Fröhlich, Martin Bertau, Giuseppe Zanoni, Maurizio Benaglia,
ChemSusChem 2024.
https://doi.org/10.1002/cssc.202401787
Alessandra Puglisi is Associate Professor in the Department of Chemistry at the Università degli Studi di Milano, Italy. Her main scientific interests lie in stereoselective organocatalysis, also in combination with photocatalysis, and the application of new enabling technologies such as flow chemistry for the development of efficient and sustainable processes of high-value (chiral) molecules.