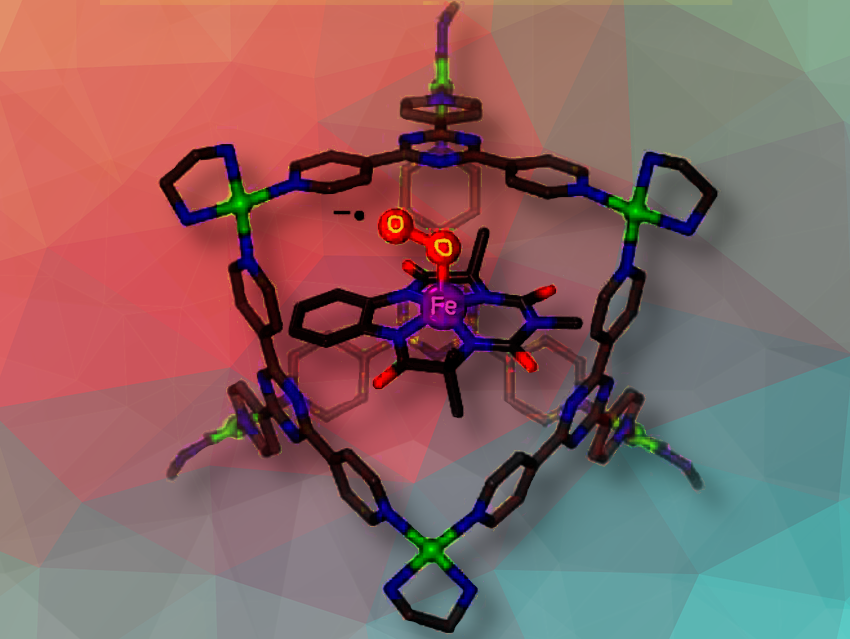
Sayam Sen Gupta, Indian Institute of Science Education and Research Kolkata, Mohanpur, West Bengal, India, Jyotishman Dasgupta, Tata Institute of Fundamental Research, Mumbai, India, and colleagues have synthesized a rare yet stable Fe(IV)-superoxo intermediate at room temperature. The team first encapsulated an (Et4N)2[FeIII(Cl)(bTAML)] complex within the hydrophobic interior of a water-soluble octahedral Pd6L412+ nanocage. The dioxygen interacts with the Fe(III) site of the complex and the rare and stable Fe(IV)-superoxo intermediate is formed.
The encapsulated species were characterized through a variety of techniques including UV-vis spectroscopy, electron paramagnetic resonance (EPR), and Mössbauer spectroscopy, which collectively confirmed the presence of a stable Fe(IV)-O2•− complex inside the nanocage in water for the first time at room temerature. The cage-encapsulated complex has a short Fe–O bond distance of ∼1.70 Å. The O2 reaction is in confinement reversible, while the formed Fe(IV)-superoxo complex readily reacts when presented with substrates having weak C–H bonds, highlighting the lability of the O–O bond.
This approach provides insights into iron-based catalysis and suggests that molecular confinement could be a useful tool for studying reactive intermediates in a controlled manner. This discovery is important because it demonstrates the stabilization of a rare Fe(IV)-superoxo intermediate, which can provide insights into the reactivity and mechanisms of oxygen activation in metalloenzymes and synthetic catalysts, potentially leading to advancements in catalysis and environmental chemistry.
- Trapping an Elusive Fe(IV)-Superoxo Intermediate Inside a Self-Assembled Nanocage in Water at Room Temperature,
Rahul Gera, Puja De, Kundan K. Singh, Sergio A. V. Jannuzzi, Aisworika Mohanty, Lucia Velasco Kulbir, Pankaj Kumar, J. F. Marco, Kalaivanan Nagarajan, Carlos Pecharromán, P. M. Rodríguez-Pascual, Serena DeBeer, Dooshaye Moonshiram, Sayam Sen Gupta, Jyotishman Dasgupta,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c05849