Iron usually has coordination numbers of 4 or 6 in its stable ionic compounds due to its small ionic radii. Under high pressure, some compounds can show hypercoordination, and studying such hypercoordinated phases can be interesting, e.g., for understanding their bonding characteristics. Eight-coordinated iron has been observed in oxides under high pressures, but the synthesis of other hypercoordinated iron compounds with different anions has remained challenging. Due to the small radius of fluoride anions, compressed Fe-F compounds are promising in the search for eight-coordinated iron compounds.
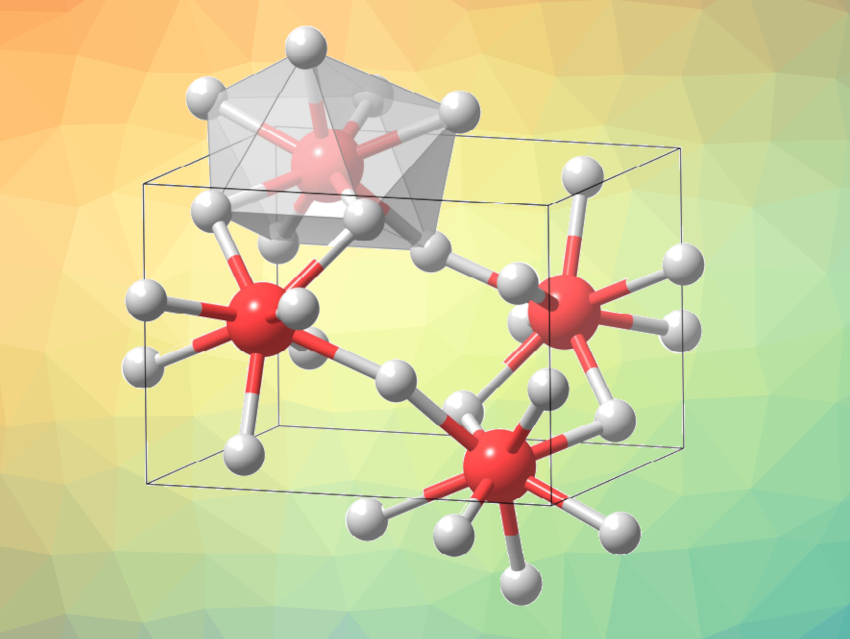
Guangtao Liu, Hanyu Liu, Yanming Ma, Jilin University, Changchun, China, and colleagues have identified a stable orthorhombic phase of FeF3 with fully eight-coordinated iron at high pressure. Using computational methods, the team predicted that a FeF3 phase with exclusively eight-coordinated iron should be energetically stable above 18 GPa. They then used a laser-heated diamond anvil cell to investigate the crystal structures of FeF3 at high pressures and temperatures.
The researchers successfully synthesized the predicted orthorhombic phase of FeF3. The structure was confirmed using in-situ X-ray diffraction. The team found a phase transition from R3c-FeF3 to eight-coordinated Pnma-FeF3 (pictured) at either 46 GPa and 2,400 K or 60 GPa and 2,300 K. They attribute the formation of Pnma-FeF3 to a reduction in volume that allows for denser structure packing. Overall, the work provides insights for the design and discovery of new hypercoordinated compounds.
- Observation of Iron with Eight Coordination in Iron Trifluoride under High Pressure,
Wencheng Lu, Siyu Liu, Mi Zhou, Hongbo Wang, Guangtao Liu, Hanyu Liu, Yanming Ma,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202319320