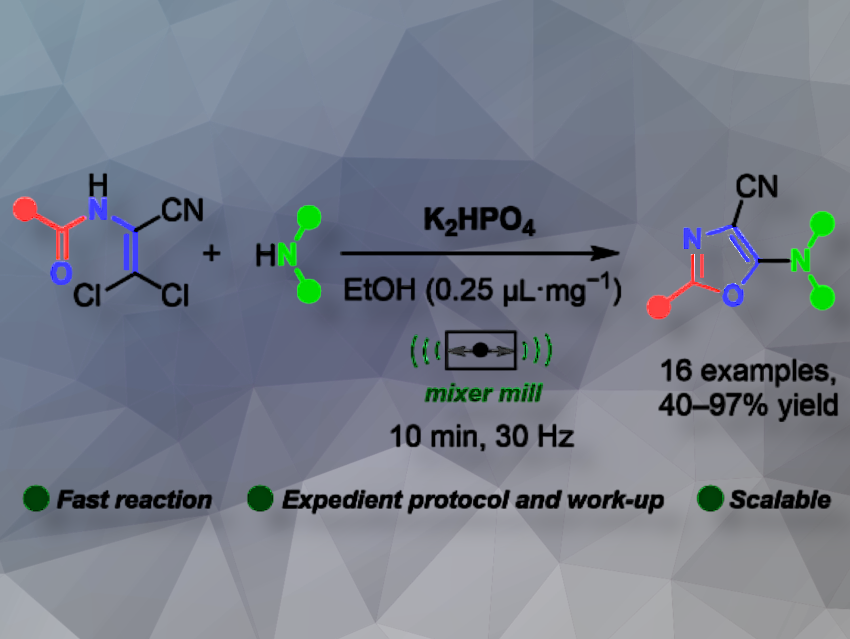
Mechanochemistry is emerging as a sustainable approach in organic synthesis, minimizing solvent use by grinding reactants in devices like ball mills. However, its application remains limited, particularly in synthesizing nitrogen-containing heterocycles such as oxazoles.
Danylo Merzhyievskyi, Dzmitry Kananovich and Riina Aav, Tallinn University of Technology, Estonia, Volodymyr Brovarets, Institute of Bioorganic Chemistry and Petrochemistry NAS of Ukraine, and colleagues have developed a mechanochemical method for synthesizing 5-amino-4-cyanoxazoles. By milling 3-amino-3,3-dichloroacrylonitriles with amines or amine salts in the presence of dipotassium phosphate, the team obtained the target compounds in good to excellent yields within just 10 minutes. Dipotassium phosphate serves as a cost-efficient and non-toxic inorganic base, ensuring 100% carbon economy of the process.
In solution, this reaction has been known for decades and is considered mature, leaving little room for improvement. However, mechanochemistry pushes the boundaries further: the method is faster, avoids excess amine by using a cheap inorganic base, and simplifies work-up. Challenges remain with less nucleophilic amines: Aromatic amines show low reactivity at room temperature. For instance, indoline achieves only 43% conversion to its oxazole product after 3 hours.
However, this work highlights mechanochemistry’s potential to expand access to valuable heterocycles, which still require further development
- Mechanochemical Synthesis of 5‐Amino‐4‐Cyanoxazoles,
Danylo Merzhyievskyi, Oleh V. Shablykin, Tatsiana Jarg, Volodymyr S. Brovarets, Riina Aav, Dzmitry Kananovich,
European Journal of Organic Chemistry 2025.
https://doi.org/10.1002/ejoc.202500145