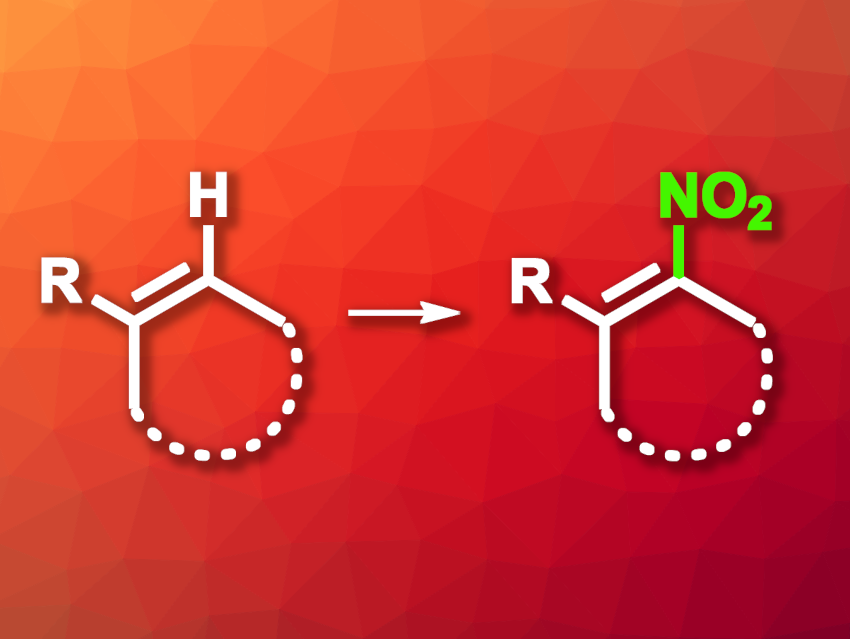
Nitroalkenes are useful intermediates in organic synthesis. They can be prepared, e.g., via the nitration of alkenes using reagents such as gaseous NO and NO2 or metal nitrates/nitrites as nitro sources along with oxidants. However, these existing methods can have some drawbacks, e.g., low selectivity or limited functional group tolerance.
Zhong-Quan Liu, Nanjing University of Chinese Medicine, China, and colleagues have developed a method for the radical nitration of alkenes using NaNO2, mediated by I2O5, to obtain nitroalkenes (general reaction pictured). The team proposed that I2O5 can serve as a green and safe single-electron oxidant to generate NO2 radicals from NaNO2. They reacted a variety of alkenes with NaNO2 in the presence of I2O5, using a CH2Cl2/H2O mixture as the solvent. The reactions were performed at room temperature.
Using this approach, the desired nitroalkenes were obtained in good to high yields. The team proposes a mechanism that involves a single-electron oxidation of NO2– to give an NO2 radical, which reacts with the alkene to give a carbon-centered radical intermediate. A deprotonation step and another single-electron oxidation then give the nitroalkene. According to the researchers, the method provides a mild, safe, and environmentally friendly path to a broad range of nitroalkenes.
- A Free Radical Nitration of Olefins with NaNO2/I2O5,
Xuan Huang, Huichao You, Fang Fang, Fan Wang, Zhongquan Liu,
Chem. Commun. 2023.
https://doi.org/10.1039/D3CC04275H