Sulfonyl fluorides are useful, e.g., in organic synthesis, agrochemistry, or pharmaceutical chemistry. Allyl sulfonyl fluorides, for example, can serve as versatile intermediates, but existing methods for their synthesis generally require multiple steps and/or harsh reaction conditions.
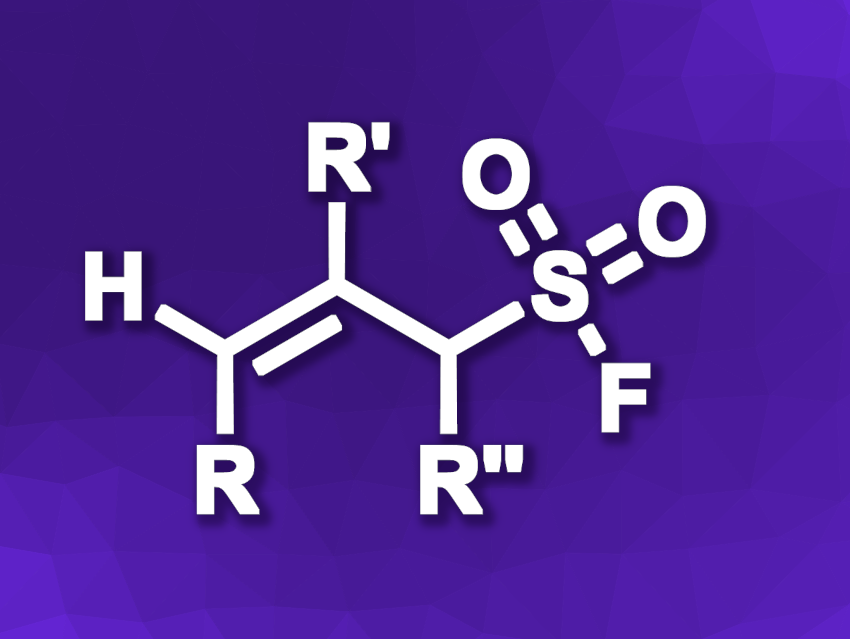
Shenlin Huang, Nanjing Forestry University, China, and colleagues have developed an approach for the electrochemical, catalyst-free radical fluorosulfonylation of allyl bromides under mild conditions (general product structure pictured). The team reacted a variety of allyl bromides with FSO2Cl using a graphite felt anode, a zinc cathode, lithium triflate (LiOTf) as an electroyte, and diethylether (Et2O) as the solvent. The reactions were performed under constant cell voltage conditions at room temperature.
Under these conditions, the desired fluorosulfonylated products were obtained in moderate to good yields. The researchers found that an allyl chloride can also serve as a suitable substrate, and they performed an up-scaled reaction on a gram scale, obtaining the product in a yield of 71 %. The allylsulfonyl fluorides can be used in further transformations.
The team proposes a reaction mechanism that involves the formation of an FSO2 radical via the electrochemical reduction of FSO2Cl. The radical undergoes an addition to the allyl bromide, and the resulting alkyl radical is converted to the product under loss of a bromo radical. Overall, the work provides straightforward access to various allyl sulfonyl fluorides.
- Electrochemical Radical Fluorosulfonylation of Allyl Bromides,
Bingcong Liu, Hui Liang, Yanju Lu, Shenlin Huang,
Org. Lett. 2025.
https://doi.org/10.1021/acs.orglett.5c00256



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)