Transition-metal-catalyzed C–C coupling reactions are widely used in organic synthesis. Nevertheless, the construction of sterically crowded quarternary carbon centers using these reactions can still be challenging. Methods for this type of transformation would be useful, since such centers are found in many natural products and pharmaceutically active compounds, for example.
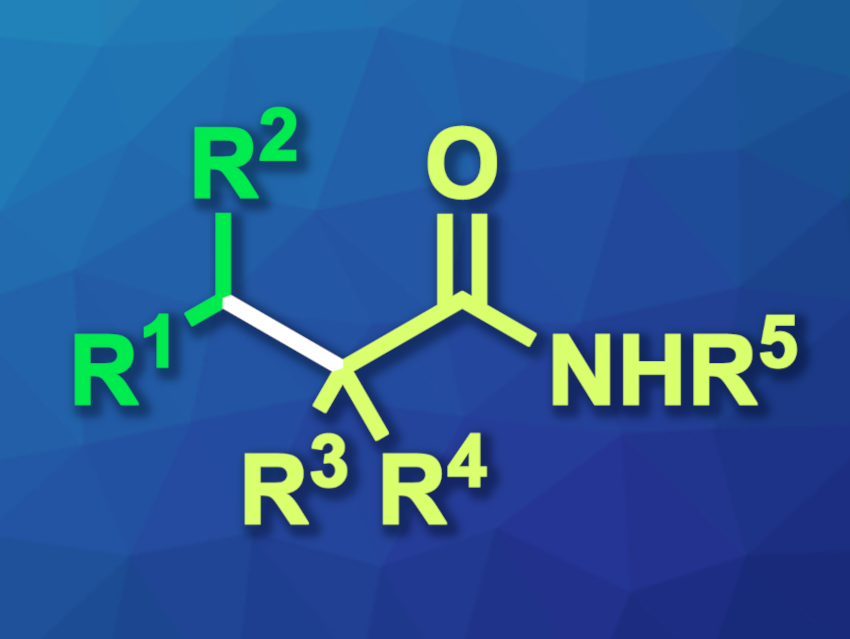
Shou-Fei Zhu, Nankai University, Tianjin, China, and colleagues have developed an approach to the coupling of tertiary alkyl halides with secondary alkyl zinc reagents using iron catalysis. The reaction gives coupling products with sterically crowded quaternary carbon centers (general structure pictured) and represents a rare example of a coupling between tertiary C(sp3) and secondary C(sp3) centers.
The team used Grignard reagents, which were transmetalated using ZnCl2 to generate the desired alkyl zinc reagents in situ, and reacted them with α-halogenated alkyl amides in the presence of an iron complex with a bidentate phosphine ligand (Fe(BF-Phos)Cl2) as the catalyst and LiCl as an additive, using tetrahydrofuran (THF) as the solvent.
The desired coupling products were obtained in moderate to good yields. The team proposes a reaction mechanism that involves the formation of an Fe(0) species from the catalyst, which then forms a complex with the alkyl amide. This is followed by a single-electron transfer (SET) to give a radical intermediate that reacts with the alkyl zinc reagent. Another SET step and a reductive elimination then give the product. Overall, the method provides efficient access to all-carbon quaternary carbon centers using a cost-effective iron catalyst.
- Iron-Catalyzed C(sp3)–C(sp3) Coupling to Construct Quaternary Carbon Centers,
Qiao Zhang, Xiang-Yu Liu, Yan-Dong Zhang, Ming-Yao Huang, Xin-Yu Zhang, Shou-Fei Zhu,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c14032