Qubits are the basic building blocks of information processing in quantum technology. An important research question is what material they will be made of in technical applications. Molecular spin qubits are seen as promising qubit candidates for molecular spintronics, especially for quantum sensing. Molecular spin qubits are molecular systems where the intrinsic spin of unpaired electrons can be used to encode and process quantum information. These qubits use the well-defined spin states of molecules, which can be manipulated using external magnetic or electric fields. Their coherence properties—how long quantum information can be retained—depend on factors like molecular structure, local environment, and spin-orbit interactions.
Molecular spin qubits have the advantage of synthetic flexibility and the ability to be tailored to specific applications. Among them, chromophore-radical systems are seen as attractive candidates. These systems can be initiated by light to form triplet-radical pairs, which can lead to the formation of quartet states by spin mixing.
Until now, research has assumed that the interaction between two spin centers (a triplet-radical pair to undergo spin mixing) can only be strong enough for successful quartet formation if the centers are covalently linked. Due to the high effort required to synthesize covalently bonded networks of such systems, their use in application-related developments in the field of quantum technology is severely limited.
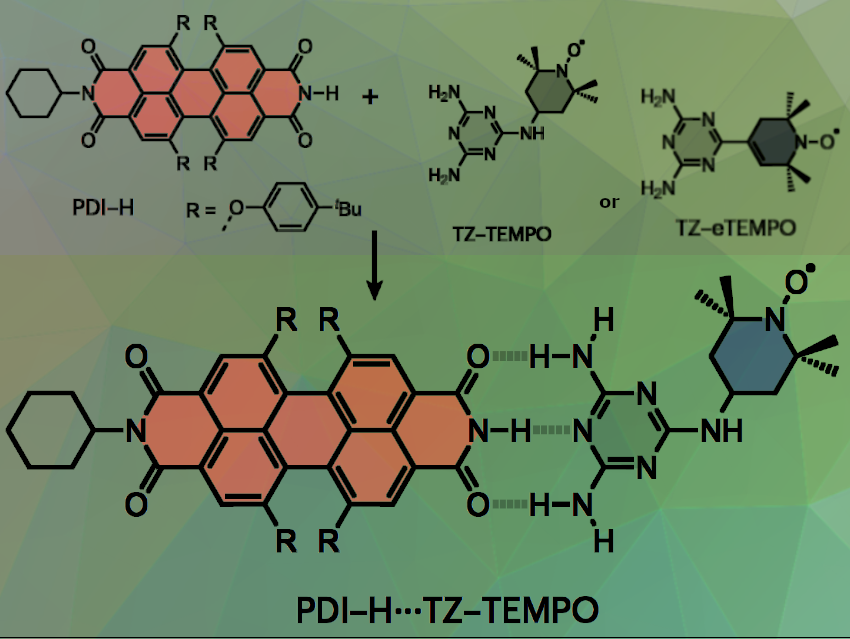
Sabine Richert, University of Freiburg, Germany, Andreas Vargas Jentzsch, Université de Strasbourg, CNRS, France, and colleagues have introduced a triple-hydrogen-bonding motif as a bridging unit to investigate whether, and to what extent, spin communication in photoexcited chromophore–radical systems can be promoted by non-covalent bonds. The team used a perylene diimide (PDI, 1,6,7,12-tetrakis(4′-tert-butylphenoxy)perylene-3,4:9,10-bis(dicarboximide) as a chromophore and nitroxide radicals (diaminotriazines functionalized with a 2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) radicals) to self-assemble in solution into supramolecular chromophore–radical systems (pictured above).
After photoexcitation, these dyads form triplet–radical pairs in which the triplet and radical spin centers communicate effectively through the triple-hydrogen-bonded bridge. In both cases, the researchers found evidence for the formation of quartet states via spin mixing using Time-Resolved Electron Paramagnetic Resonance (trEPR) spectroscopy, which was further confirmed by transient nutation experiments.
The team found a stronger coupling in PDI–H···TZ–eTEMPO than in PDI–H···TZ–TEMPO, despite its weaker
association constant in solution which they attribute to the unsaturation in the piperidine ring in combination with a more planar geometry and a slightly decreased distance between the chromophore and the radical.
This means the formation of an ordered network of spin qubits could now be achieved using supramolecular approaches, which would enable the testing of new molecule combinations and system scalability without major synthetic effort. The results show that hydrogen bonding can allow spin mixing, leading to the formation of high-spin species. Moreover, while this study was limited to triplet–radical systems, the researchers anticipate the suitability of hydrogen-bonding bridges to enable effective spin communication to be applicable to other putative molecular spin qubit candidates such as spin-correlated radical pairs and coordinated metal complexes.
According to the researchers, this finding that non-covalent bonds can enable spin mixing advances supramolecular chemistry as a valuable tool for exploring, developing, and scaling up materials for quantum information science.
- Supramolecular dyads as photogenerated qubit candidates,
Ivan V. Khariushin, Philipp Thielert, Elisa Zöllner, Maximilian Mayländer, Theresia Quintes, Sabine Richert, Andreas Vargas Jentzsch,
Nature Chemistry 2025.
https://doi.org/10.1038/s41557-024-01716-5