The production of H2 from water, driven by sunlight, could provide sustainable, renewable fuel. This reaction needs suitable catalytic systems, but most existing catalysts work only with high-energy light regions, which limits the overall efficiency. Red-light-driven H2 production is possible, but existing catalytic systems for this are generally based on precious metals, while catalysts based on abundant elements are rare and can have limited activity and stabilty.
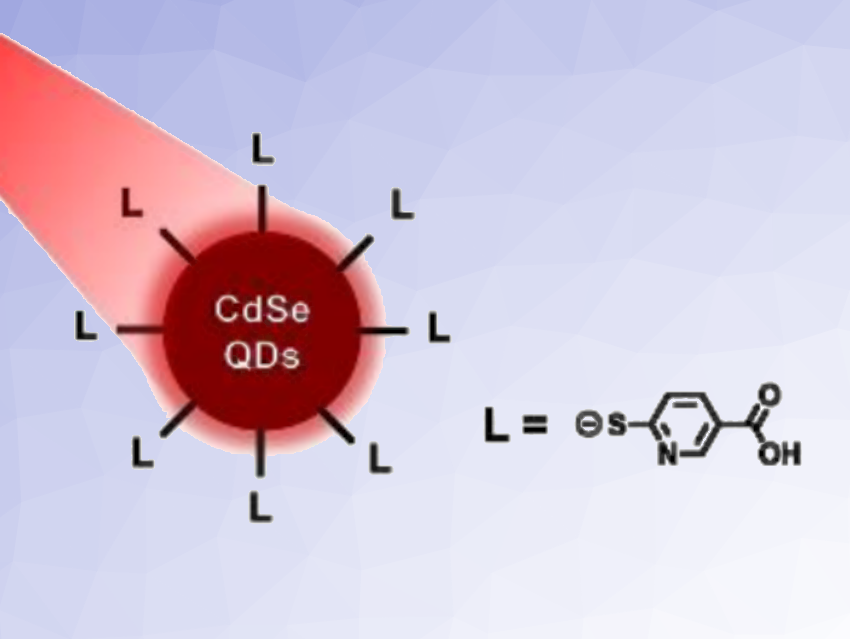
Zhiji Han, Sun Yat-sen University, Guangzhou, China, and colleagues have prepared red-light absorbing, pyridinethiolate carboxylic acid (pyS-COOH)-capped CdSe quantum dots (pictured schematically, L = pyS-COOH), which can be used for H2 production in aqueous media. Quantum dots are finely dispersed nanoscopic crystals of inorganic semiconductors. The team prepared CdSe quantum dots capped with dihydrolipoic acid (DHPA), 3-mercaptopropionic acid (MPA), mercaptobenzoic acid (pS-COOH), or pyS-COOH for comparison purposes. The differently capped quantum dots were then tested for their photocatalytic activity for H2 generation under red light in the presence of Ni(NO3)2 and ascorbic acid in aqueous solutions.
The team found that negligible amounts of H2 were produced using the DHLA- and MPA-capped quantum dots. The pS-COOH-capped system generated some H2. The researchers proposed that the introduction of a pyridyl group into the thiolate capping ligand could result in higher activity for H2 generation, and pyS-COOH-capped quantum dots indeed showed a much higher activity. The system showed a high turnover number (TON) of ca. 43910 and a H2 evolution rate of ca. 31570 μmol g–1 h–1.
- Pyridinethiolate‐Capped CdSe Quantum Dots for Red‐Light‐Driven H2 Production in Water,
Zuting Wei, Shuang Yang, Jingxiang Lei, Kai Guo, Huiqing Yuan, Mei Ming, Jiehao Du, Zhiji Han,
Chem. Eur. J. 2024.
https://doi.org/10.1002/chem.202401475