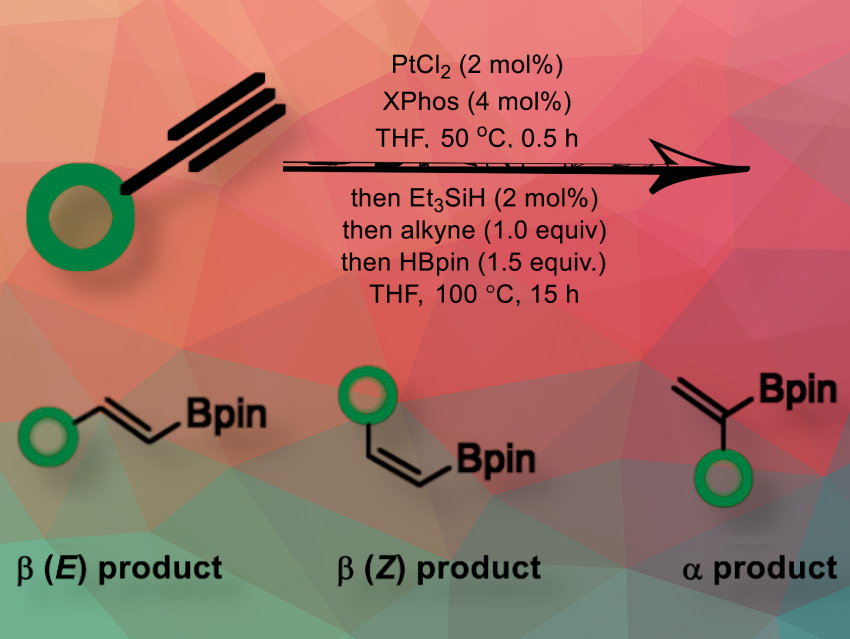
Vinyl boronates are widely used in medicinal and materials chemistry, which is why new methods for their synthesis are important, especially those that are efficient and do not require special conditions and rare catalysts. Existing hydroboration methods for vinyl boronate synthesis often face issues with heteroatom coordination, which can reduce regioselectivity and lead to inseparable product mixtures. Therefore, a general vinyl boronate synthesis from alkynes remains an important goal, especially a hydroboration method compatible with multiple functional groups.
Mark Gerard McLaughlin, Queen’s University Belfast School of Chemistry and Chemical Engineering, UK, and colleagues have developed a PtCl₂/XPhos/Et₃SiH-catalyzed hydroboration method for unactivated terminal alkynes. It provides an operationally simple and general method to produce vinyl boronates in good to excellent yields and regioselectivity. The reaction proceeds in hindered alkyne systems and works well on acetylenes that contain multiple heteroatom-groups to provide the desired (E)-1,2-vinyl boronates. In particular, it hydroborates
terminal alkynes derived from N-aromatic heterocycles; a class of terminal alkynes that other hydroboration methods do not successfully hydroborate with HBpin (Pinacolborane or 4,4,5,5-Tetramethyl-1,3,2-dioxaborolane).
The researchers reacted phenylacetylene with HBpin under the previously optimized catalytic conditions of PtCl₂, XPhos, and Et₃SiH in THF.
The protocol is tolerant of a wide range of diverse functional groups which include silyl ethers, benzyl and p-methoxybenzyl ethers, acyl esters, acetals, phenylsulfones, O-tosylate esters, divalent sulfides, nitro-functionalized substrates, and alkenes. As such, it is capable of providing structurally diverse vinyl boronates. The method does not reduce many readily reducible functional groups such as nitro, ester, or sulfonate esters and leaves O-isopropylidene acetals intact. The researchers emphasize also its operational simplicity, and its use of stable, readily-accessible, commercial catalysts and reagents.
The team is in the process of clarifying the role of Et₃SiH in the reaction mechanism. So far they assume that the reaction follows a Chalc-Harrod pathway, rapidly reducing the initially formed PtCl₂(XPhos)₂ to Pt⁰(XPhos)₂. This then undergoes oxidative addition with HBpin, followed by a series of steps involving α-complexation with the alkyne, migratory insertion, and reductive elimination, ultimately producing the vinyl boronate and regenerating the catalyst Pt⁰(XPhos)₂ for the next cycle.
- An Operationally Simple, Regioselective, Platinum-Catalyzed Hydroboration of Unactivated Alkynes,
K. Lawrence E. Hale, Dean D. Roberts, Mark Gerard McLaughlin,
Eur. J. Org. Chem. 2025.
https://doi.org/10.1002/ejoc.202401355