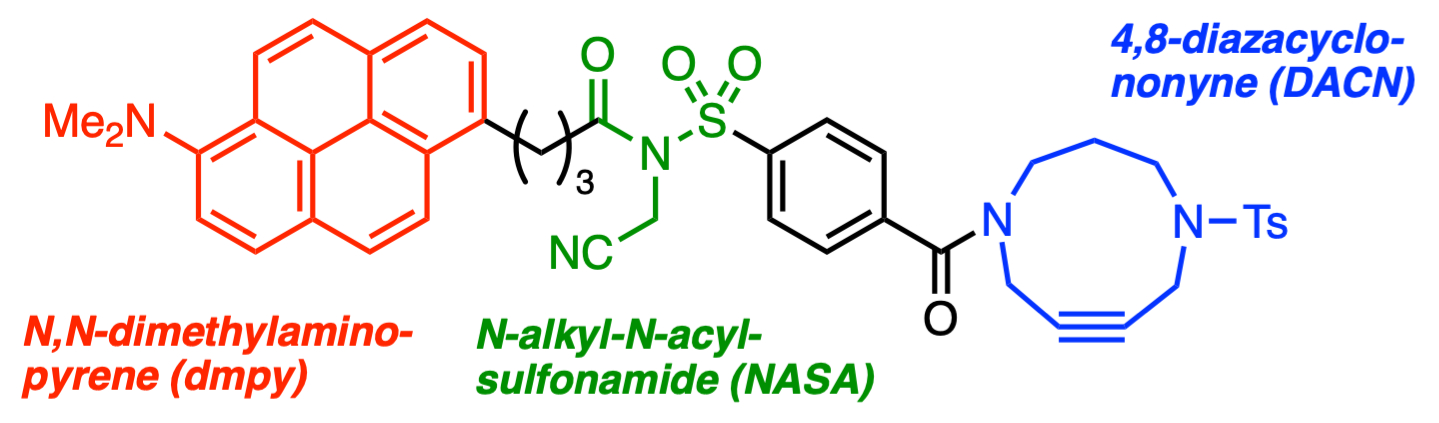
The specific chemical labeling and detection of biomacromolecules, such as proteins, polysaccharides, and nucleic acids, is important for understanding phenomena in living organisms. It can also be useful for the development of tools for the diagnosis and treatment of diseases. 6-N,N-Dimethylaminopyrene (dmpy, pictured below in red), for example, is a versatile fluorescent tag.
Masaki Kita, Nagoya University, Japan, and colleagues have developed a practical method for the in situ labeling of target proteins. For this, the team synthesized a dmpy–N-acyl-N-alkylsulfonamide–4,8-diazacyclononyne (dmpy–NASA–DACN) tag (pictured below). The tag was prepared starting from N-Ts-DACNamine hydrochloride and 4-sulfamoylbenzoic acid, which were combined using a condensation reaction, followed by a reaction with dmpy-COOH and an N-alkylation.
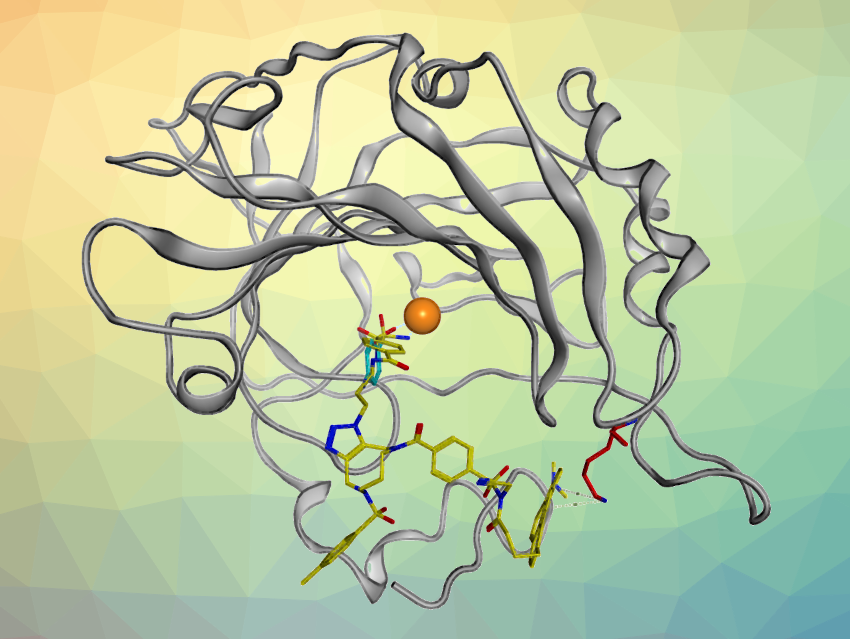
This tag was then incorporated into several dmpy-based probes by binding it to azide-functionalized ligands via an azide-alkyne cyclization. These ligands target specific proteins. Using the resulting probes, the respective target proteins were specifically labeled under conditions that mimic cellular environments. The work could contribute to the development of chemical probes for the site-specific labeling of cellular target molecules, and to the analysis of the precise binding modes of various biomacromolecule–ligand systems.
- Ligand‐Dissociation‐Type N,N‐Dimethylaminopyrene Probes for In‐Situ Site‐Specific Protein Labeling,
Tomohito Tsuda, Atsushi Arai, Masaki Kita,
Chem. Asian J. 2022.
https://doi.org/10.1002/asia.202200631